Fig. 4.1
Coronal image shows a colovaginal fistula (arrow) in a woman who has previously had a hysterectomy
Colocutaneous fistulas rarely occur de novo and are generally associated with a leak from a prior anastomosis or with a prior percutaneous drain. In a large series of colocutaneous fistulas, leaving sigmoid colon distal to an anastomosis (i.e., not resecting the entire sigmoid colon) was a risk factor for the development of a fistula [45].
Diverticular Stricture
Key Concept: Symptomatic diverticular strictures should be resected; mucosal evaluation to exclude other diagnoses such as malignancy, IBD, or ischemia should be performed.
Strictures or partial obstruction may also occur in association with multiple attacks of diverticulitis. Many patients do not present with a complete large bowel obstruction but rather with progressive constipation and obstructive symptoms. Once again, endoscopic visualization of the mucosa is helpful to exclude other diagnoses such as colon cancer, a stricture resulting from ischemic colitis or from inflammatory bowel disease. Colonic stenting can be considered for patients with large bowel obstruction from diverticulitis with an aim toward stabilizing the patient, decompressing the bowel, and ultimately performing a single-stage sigmoid resection. Our success rate is poor for stenting diverticular disease and much better for stenting for obstructing cancer.
Diverticular Abscess
Key Concept: Image–guided percutaneous drainage is usually the most appropriate treatment for patients with large diverticular abscesses and does not necessarily require subsequent surgical resection.
Diverticulitis may be associated with an abscess in a small percent of cases. Approximately 10 % of patients hospitalized for diverticulitis at the Lahey Clinic have an associated abscess. Data from the Nationwide Inpatient Sample, the largest all-payer database of discharged patients in the country, shows that the incidence of diverticular abscess has increased from 5.9 % in 1995 to 9.6 % in 2005 [46]. The increasing numbers of patients with diverticular abscess may be related to the widespread and increasing usage of CT scanning for the initial diagnosis of diverticulitis. A number of staging systems have been utilized, but most commonly the Hinchey classification (with modifications thereof) is utilized. Stage 0 = mild clinical diverticulitis; stage 1a = confined pericolic inflammation/phlegmon; stage 1b = pericolic abscess; stage II = pelvic, intra-abdominal, or retrocolic abscess; stage III = purulent peritonitis; and stage IV = fecal peritonitis [47]. Many small abscesses (defined as those <4 cm) may be treated with antibiotics with successful resolution and do not require percutaneous drainage or repetitive CT scans (especially if the patient is clinically responding with decrease in pain, fever, and leukocytosis) (Fig. 4.2) [48]. Combined series have shown that initial treatment with antibiotics (with or without percutaneous drainage) is successful in 30–56 % of patients [49]. Percutaneous drainage was initially used as a bridge to surgery; patients underwent drainage, sepsis resolved, and surgery was then performed electively [50]. Currently, percutaneous drainage is also used as definitive therapy, and some patients may not have further symptoms following successful resolution of the abscess. The decision to perform subsequent resection may therefore be made on an individual basis, recognizing patients with abscess have more severe diverticulitis and are more likely to require surgery. In rare cases, laparoscopic drainage may be performed if there is no radiologic window to drain an abscess (Video 4.1). The location of the abscess has been shown to help determine the clinical course, as those patients with more distant abscesses (i.e., Hinchey stage II) are more likely to require resection than patients with pericolic abscess. In a cohort of 465 patients, 73 patients (17 %) had an abscess, of which 45 patients had a pericolic abscess and 28 patients had a pelvic abscess. A larger number of patients with pelvic abscess (71 %) required surgery compared to those with pericolic abscess (51 %) [51].
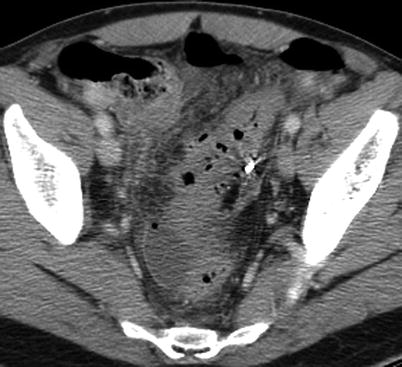
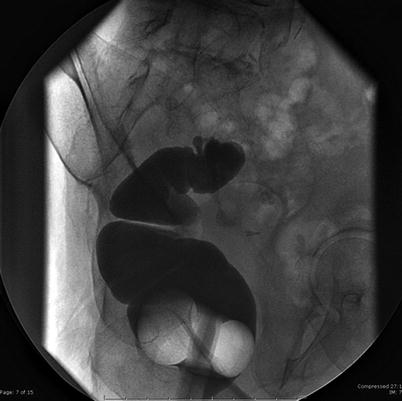

Fig. 4.2
Pelvic abscess (Hinchey II) with foci of free air

Fig. 4.3
Gastrografin enema shows residual sigmoid colon and diverticula
Perforated Diverticulitis with Purulent or Feculent Peritonitis
Key Concept: The traditional therapy of mandatory colonic resection with or without diversion for perforated diverticulitis continues to evolve with the development of improved imaging, antibiotic success, endoscopic techniques, and implementation of laparoscopic lavage.
The optimal treatment for perforated diverticulitis and associated peritonitis continues to evolve. Options include Hartmann resection, sigmoid resection with primary anastomosis (in selected patients), sigmoid resection and primary anastomosis with proximal diversion, on-table lavage with primary anastomosis, and laparoscopic lavage without resection. Hartmann resection remains one of the most common operations performed for perforated diverticulitis but has a number of drawbacks. Approximately 30 % of patients never undergo reversal of the stoma [52, 53]. In addition, the operation has considerable morbidity and a reported mortality of up to 18.8 % [54]. Over the years, a number of other options have been advocated. Fibrin glue with suture repair and omental patching of the perforation has been reported [55]. Two studies (both underpowered) looked at the role of defunctioning the diseased segment with suture of the perforation and proximal diversion versus resection and had different conclusions [56, 57]. The role of on-table lavage in approaching patients with colonic emergencies has largely fallen out of favor since the need for bowel preparation has been challenged by a number of reviews [58]. A systematic review of 569 cases in 50 studies suggested that primary anastomosis with or without diversion was “safe in certain patients with peritonitis” but noted a mortality of 9.9 % and an anastomotic leak rate of 13.9 % [54].
In approaching the patient with perforated diverticulitis, it is important to distinguish between patients who have evidence of peritonitis on physical examination and those patients who have CT findings consistent with perforation but no objective findings of toxicity. While many surgeons trained in the 1970s or 1980s were taught that the finding of free air on a chest x-ray or KUB was an absolute indication for surgery, the findings of free air on CT imaging do not necessarily translate into similar recommendations. Dharmarajan and coworkers evaluated CT findings of perforated diverticulitis and devised a grading system based on the amount and location of abnormal air, which may assist with clinical decision-making [59]. While a grading system is a useful adjunct, I personally rely more heavily on the clinical status of the patient and base my initial strategy on the physical examination findings more than the CT findings alone.
There has recently been a renewed interest in the role of laparoscopic lavage without resection for patients with perforated diverticulitis and associated purulent peritonitis. In 1996, O’Sullivan and colleagues reported 8 patients with perforated diverticulitis and purulent peritonitis who underwent a laparoscopic lavage [60]. No resection of the sigmoid colon was performed, and patients were subsequently treated with intravenous antibiotics. At a follow-up of 12–48 months, no patient required subsequent resection, and no patient required an emergent colostomy. Based on these initial encouraging results, a prospective multi-institutional trial was subsequently performed of 100 patients with perforated diverticulitis who underwent laparoscopic lavage [61]. The median age was 62.5 years, and patients were followed for 36 months. The procedure was performed with an umbilical, suprapubic, and right lower quadrant ports, and patients were lavaged with 4 l of fluid or lavaged until the returns were clear. Eight out of the 100 patients were noted to have fecal peritonitis and were converted to an open procedure and underwent resection and stoma. Of the 92 patients who were managed with laparoscopic lavage, no patient required subsequent resection for diverticulitis at a median follow-up of 36 months. There was an overall 4 % morbidity and 3 % mortality rate for the cohort. Two patients developed a pelvic abscess and required drainage, while 2 patients presented with a subsequent attack of diverticulitis. The authors concluded that laparoscopic lavage was a reasonable alternative with low mortality and low morbidity, particularly when compared with Hartmann resection. Furthermore, they suggested that elective resection, even in this group of patients who presented with perforation, was probably unnecessary and that readmission was uncommon.
Currently, the role of laparoscopic lavage continues to evolve in the treatment of patients with perforated diverticulitis and associated purulent peritonitis. A number of additional small series have been reported, including a recent review article evaluating 12 nonrandomized studies encompassing 301 patients with a mean age of 57 years [62]. Although the majority of patients in these combined series had Hinchey III classification (i.e., purulent peritonitis), 25 % of patients had Hinchey II disease. In the Myers series, 25 % of patients also had Hinchey II disease, suggesting that some of these patients could potentially have been treated with bowel rest and antibiotics alone, along with subsequent percutaneous drainage for those patients developing abscesses [61]. In this combined series, the conversion rate was 4.9 %, while the mean complication rate was 18.9 % and mortality was 0.25 %. Subsequent resection was performed in 51 % of patients, and the majority of the resections were laparoscopic. In the future, we need to identify those patients who may optimally be treated by lavage. Further classification of the degree of peritonitis either by the Mannheim peritonitis index or the peritonitis severity score may help to further define the optimal candidate for lavage. Similarly, the need for subsequent resection has not been defined. In the Afshar series, the majority of patients who underwent elective resection did so because of surgeon preference [62]. It goes without saying that colonoscopic evaluation of the colon is important in patients to exclude a diagnosis of perforated colon cancer.
A number of guidelines have been refined to include a statement on lavage. The European Association for Endoscopic Surgery consensus statement of laparoscopy for abdominal emergencies states that “colon resection remains the gold standard, but laparoscopic lavage and drainage may be considered in some selected patients” [16]. The Association of Coloproctology of Great Britain and Ireland states that “laparoscopic lavage may play a role in some patients with acute diverticulitis. Whilst this is an alternative to resection in the acute setting for some patients, it is not certain whether it is an acute alternative to delayed resection” [12]. At the present time, I use laparoscopic lavage selectively in otherwise fit patients with perforated diverticulitis. In the concept of the calculated risk, we as the surgeons “make the calculations,” and the patients “incur the potential risk.” I do not generally recommend lavage to unstable patients or those with a number of other associated comorbidities.
Reoperative Surgery for Diverticular Disease
Key Concept: Reoperative surgery entails unique technical and decision–making challenges that need to be considered both prior to and at the time of surgery to optimize outcomes.
Reoperation for complicated diverticular disease occurs for two main reasons: as a planned procedure to restore intestinal continuity after resection, stoma, and Hartmann closure of the rectum and as an unplanned procedure to treat complications or unanticipated events after initial resection and primary anastomosis. The latter occurrence is mainly due to anastomotic leakage but may occur from fistula, abscess, or stricture at the anastomosis. This section discusses considerations prior to reoperative surgery including anatomy, timing of reoperation, anatomic considerations, preoperative preparation, conduct of the operation, and outcome.
Reoperative Surgery After Hartmann Resection
The Hartmann resection was first described by Henri Hartmann for the treatment of rectal cancer in which he described two patients presenting with obstruction in whom he resected the tumor and closed the “superior part of the rectum and left it in the peritoneum without disturbing the pelvic floor” [63]. The procedure quickly became the procedure of choice for the majority of patients who underwent emergency surgery for perforated diverticulitis in the second half of the twentieth century, replacing the three-stage procedure of initial colostomy, subsequent resection, and finally colostomy takedown that was advocated by Lockhart-Mummery [64, 65]. Of note, Hartmann believed that reversal of the Hartmann procedure should not be attempted. Currently, Hartmann takedown still has significant morbidity and mortality and a relatively low reversal rate. Unfortunately, the risk of needing to return to the operating room for a repeat stoma remains high.
Timing
After Hartmann resection for perforated diverticulitis, most patients are eager to proceed as soon as possible with reversal of the colostomy. In contrast to patients who may be chronically ill with inflammatory bowel disease for years prior to resection, these patients often had never been ill before and had never anticipated leaving the hospital with a stoma after treatment for diverticulitis. Surgery for Hartmann reversal may be undertaken early (<3 months from initial surgery) or late (>3 months from initial surgery). There are advocates of each approach [66–70]. Proceeding with Hartmann takedown close to the time of initial surgery has several disadvantages, predominantly due to adhesions and the acute inflammatory response after initial surgery which may lead to a difficult dissection, potential enterotomies, and difficulty with identification of the Hartmann stump. While waiting for at least 3 months will presumably allow the patient sufficient time to heal and facilitate identification of the Hartmann stump, waiting longer may make identification of the stump more difficult secondary to fibrosis. The two approaches (waiting less than 3 months vs. greater than 3 months) have not been assessed in a randomized trial. My approach has been to wait for 3 months prior to Hartmann takedown. Waiting for this time period ideally reduces the difficulty and potential complications from adhesions.
Preoperative Preparation
General preoperative assessment of the patient should routinely be performed. Nutritional status is optimized. Cardiopulmonary disease is identified and evaluated. Reoperative pelvic surgery is associated with a high risk of thromboembolic complications, and patients are administered appropriate prophylaxis. Although increasing evidence suggests that mechanical bowel preparation is not necessary, I believe that it is preferable in reoperative surgery to minimize spillage in case the bowel is entered. Preoperative intravenous antibiotics are administered, although there is little evidence to support additional dosing.
Preoperative Imaging
For patients >50 years old who have not had prior colonic evaluation, a colonoscopy or barium enema should be performed. Prior to planning Hartmann takedown, my preference is to perform a barium enema through the stoma and a Gastrografin enema through the rectum. The Gastrografin enema is particularly useful as it gives an assessment of the length and configuration of the rectal segment and gives an assessment of any residual sigmoid colon and/or diverticula (Fig. 4.3). Many patients have undergone the initial resection by another surgeon; at times, because of intraoperative factors, a substantial amount of sigmoid colon is left in place. The road map of the specific anatomy is better determined by a Gastrografin study than by a flexible sigmoidoscopy, although both can be performed. These procedures are also helpful to evacuate retained fecal residue. Scybala retained in the rectum from the original Hartmann resection should be evacuated at this time or with distal rectal washout at the time of surgery to facilitate placement of a sizer and subsequently the EEA stapler. Even with a washout at the time of colostomy takedown, this may be difficult to accomplish.
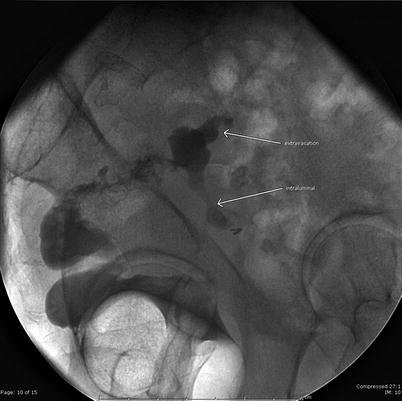

Fig. 4.4
Gastrografin enema shows a leak at the top of the Hartmann pouch with extravasation (arrow) and intraluminal contrast (arrow) into small bowel
Intraoperative Considerations
Patient Positioning
Anticipate a long procedure and pad the patient’s bony prominences accordingly. The patient may be placed in lithotomy position in Lloyd Davies, Allen, or Yellowfin stirrups. Care should be taken to avoid pressure on the peroneal nerves and the hips. Overall, my preferred position is aimed to have symmetric hip extension, knee flexion, and thigh abduction. Extreme hip extension beyond 60° can occasionally lead to femoral nerve palsies if a self-retaining retractor is positioned against the extended extremity. The perineum should be hanging slightly over the table to ensure easy passage of the EEA stapler. Rectal washout can be performed and a mushroom catheter left in the rectum if desired to facilitate identification of the Hartmann pouch. A proctoscope and/or sizer may also be used intraoperatively to identify the pouch. The vagina should also be included in the prep. Alternatively, my preference is to position the patient supine on a split leg table with the legs abducted. The split leg table avoids potential difficulties with long-standing lithotomy position including nerve injuries and compartment syndrome. Once again, care must be taken to ensure that the patient is positioned far enough down on the table that access to the anus (to pass the EEA stapler) can be achieved. A beanbag with the arms tucked at the sides can be helpful to ensure the patient does not slip cephalad on the table, especially when in steep Trendelenburg position.
Approach to the Procedure
The procedure may be undertaken by a laparoscopic or open approach. Adhesions encountered from previous surgery or prior infection may make a laparoscopic approach impossible. The extent and degree of adhesions may be difficult to predict; on occasion much less severe adhesions are encountered than anticipated, and the procedure progresses quite smoothly. Alternatively, with extensive adhesions, bowel injury may occur when attempting to enter the peritoneal cavity. A reasonable approach is the use of a “peek port” which entails entering the abdomen through a small incision and assessing the degree of adhesions [71]. The laparoscopic equipment is not opened until the feasibility of a laparoscopic hand-assisted approach is determined. Alternatively, a port can also be placed away from the site of the previous surgery to assess the degree of adhesions and the feasibility of a straight laparoscopic approach.
Exposure and Lighting
The importance of having adequate exposure and lighting cannot be overestimated with reoperative surgery. If an open approach is used, the incision should extend to the symphysis pubis. Cephalad extension of the midline incision may be needed if splenic flexure mobilization is needed. Operating between the patient’s legs provides optimal visualization of the splenic flexure as does rotation of the table to a left-side-up position.
Adequate OR lighting, a headlight, and/or lighted pelvic retractors are helpful. A self-retaining retractor with bladder blade is also used. Straight blade (Wylie renal vein or St. Mark’s) and curved (Deaver) retractors are available, with the former being more helpful for deep pelvic dissection, which is on occasion necessary to free up the Hartmann stump. Care must be taken to avoid placing these retractors on the drapes and causing a fire.
Initial Dissection
The initial dissection is focused on lysing all small bowel adhesions in the pelvis to be able to identify the Hartmann pouch. Ultimately, in the majority of cases, all small bowel adhesions from the ligament of Treitz to the ileocecal valve are lysed to be able to mobilize the colostomy and bring the proximal colon down to the pelvis without tension. The pelvic dissection associated with a prior Hartmann resection may be challenging secondary to dense adhesions and the inability to distinguish a plane suitable for dissection. It is advisable to lyse the filmy small bowel adhesions first and then attack the more difficult adhesions. With few exceptions, there are small bowel and/or omental adhesions to the top of the Hartmann pouch. Dense adhesions often occur to the top of the Hartmann pouch, and encountering staple material is an indication of proximity to this structure. If extremely dense adhesions are encountered, hydrodissection or infiltration of the fused area with saline with a small-gauge needle may be helpful [72]. The appendix can also be drawn down into the pelvis toward the Hartmann and may occasionally lead the surgeon to believe he or she has encountered the right ureter. The left ovary and tube, in particular, may be fused with the top of the Hartmann pouch. Bleeding from the pelvic wall may often occur from entering the fallopian tubes or a branch of the ovarian vessels.
The ureters should be identified, and the surgeon should be aware that they may be in an unanticipated position, particularly drawn in more medially, after prior surgery. Ureteral stents may be used in selected cases with prior severe pelvic sepsis or unclear anatomy. Stents do not prevent ureteral injury but facilitate the recognition of such injury. I selectively use stents in patients with hydronephrosis or a large amount of retroperitoneal inflammation. The vagina may be adherent to the rectum and dissection facilitated by placing a finger in the vagina to identify the proper planes.
The colostomy is mobilized by incising the mucocutaneous junction and trying to preserve all the mesenteric attachments. Injection with saline or local anesthetic around the mucocutaneous junction circumferentially may facilitate dissection. The stoma is resected and fresh bowel used for the intended anastomosis. Once the stoma is mobilized, the surgeon can generally assess whether there is adequate length for a tension-free anastomosis. Additional length is facilitated by a number of maneuvers including division of the lateral colonic attachments, takedown of the splenic flexure, division of the inferior mesenteric artery at the takeoff of the aorta, and division of the inferior mesenteric vein at the inferior border of the pancreas. Alternatively, further length can be achieved by mobilizing the rectum further distally and essentially bringing the Hartmann pouch up to the proximal bowel. Once complete mobilization of the proximal colon is performed and adhesiolysis is completed, the small bowel and colon can be packed into the upper abdomen.
Identification and Mobilization of the Hartmann Pouch
Once the small bowel is mobilized, the top of the Hartmann pouch can be identified. Some surgeons mark the top of the pouch with long suture material to facilitate identification. I have not found this to be helpful and have found that insertion of a proctoscope or flexible sigmoidoscope facilitates identification of the Hartmann pouch. The staple line of the Hartmann is identified, and the length of the pouch is usually longer than anticipated, even if it is located below the pelvic brim. If the staple line is adherent to the presacral fascia, it is generally safe to commence the dissection in the posterior midline, thus avoiding the ureters and the iliac vessels. It is not uncommon for the superior rectal artery to be left intact, and placing a Babcock clamp on the end of the Hartmann pouch and applying cephalad traction facilitate identification of the mesentery and straightening of the rectum. My practice is to mobilize and dissect out the Hartmann pouch at least to the mid- to proximal rectum. This is generally necessary to “straighten out the rectum,” which often times has a concertina-like configuration following Hartmann resection. If this is not done, it is often difficult to guide the EEA stapler per anum to the top of the Hartmann pouch. Once the Hartmann pouch is mobilized, a small sizer is placed per rectum to ensure that this passes easily to the area of the intended anastomosis. In those patients who have had significant sepsis or in those who have had a long-standing Hartmann pouch, further mobilization may be needed. We have found that in women further dissection is often needed in the anterior cul-de-sac as the mid-rectum tends to angulate and adhere to the uterus. Despite further mobilization, some patients may still have a fairly fibrotic pelvis (in which the rectum is intrinsically normal but the surrounding tissues are fibrotic enough that it is impossible to pass a sizer). In this case, an EEA-stapled anastomosis may not be feasible and a handsewn anastomosis preferable. The top of the intended site of anastomosis is then re-resected and the integrity of the rectum tested by filling the pelvis with saline and insufflating the Hartmann pouch.
Performing the Anastomosis
I prefer using the EEA stapler to perform anastomosis after Hartmann resection. The anvil is placed in the proximal bowel. A handsewn purse string is placed, or a purse-string device may be used. A sizer is used to guide through the rectum to the top of the re-resected Hartmann pouch. Occasionally, it is difficult to introduce the stapler into the anus, and Khoury and Opelka have reported placement of a Faensler or Chelsea-Eaton anoscope with gradual dilatation of the sphincter and placement of the stapler shaft through the anoscope [73]. The EEA stapler is guided through with the trocar exiting at the top of the Hartmann pouch, the anvil is snugged up and secured, and the stapler is fired. The instrument is generally removed easily, and the tissue rings are inspected for thickness and integrity. The anastomosis is then tested by occluding the bowel proximally and introducing air through a proctoscope or a flexible sigmoidoscope [74].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








