Chapter 3.20
Diverticular disease and nutrition
Santhini Jeyarajah
Kings College Hospital NHS Foundation Trust, London, UK
3.20.1 Dietary factors involved in causation
Diverticula of the colon are acquired herniations of colonic mucosa, protruding through the circular muscle at the points where the blood vessels (vasa recta) penetrate through the colonic wall. They tend to occur in rows between the strips of longitudinal muscle, sometimes partly covered by appendices epiploicae. They most commonly involve the sigmoid colon but can be pancolonic. As the rectum has a complete muscle layer it is not affected [1].
It is important to differentiate between diverticulosis and the presence of diverticula which may be asymptomatic, and clinical diverticular disease (DD) where the diverticula are causing symptoms due to disordered colonic function resulting in distension, pain and altered stool output. There are several terms used in the modern description of DD that must be clearly understood. Diverticular disease is an all-encompassing term including symptomatic and asymptomatic disease. The most commonly used terms are defined in Table 3.20.1. The evolution of normal colon to symptomatic DD is summarised in Figure 3.20.1.
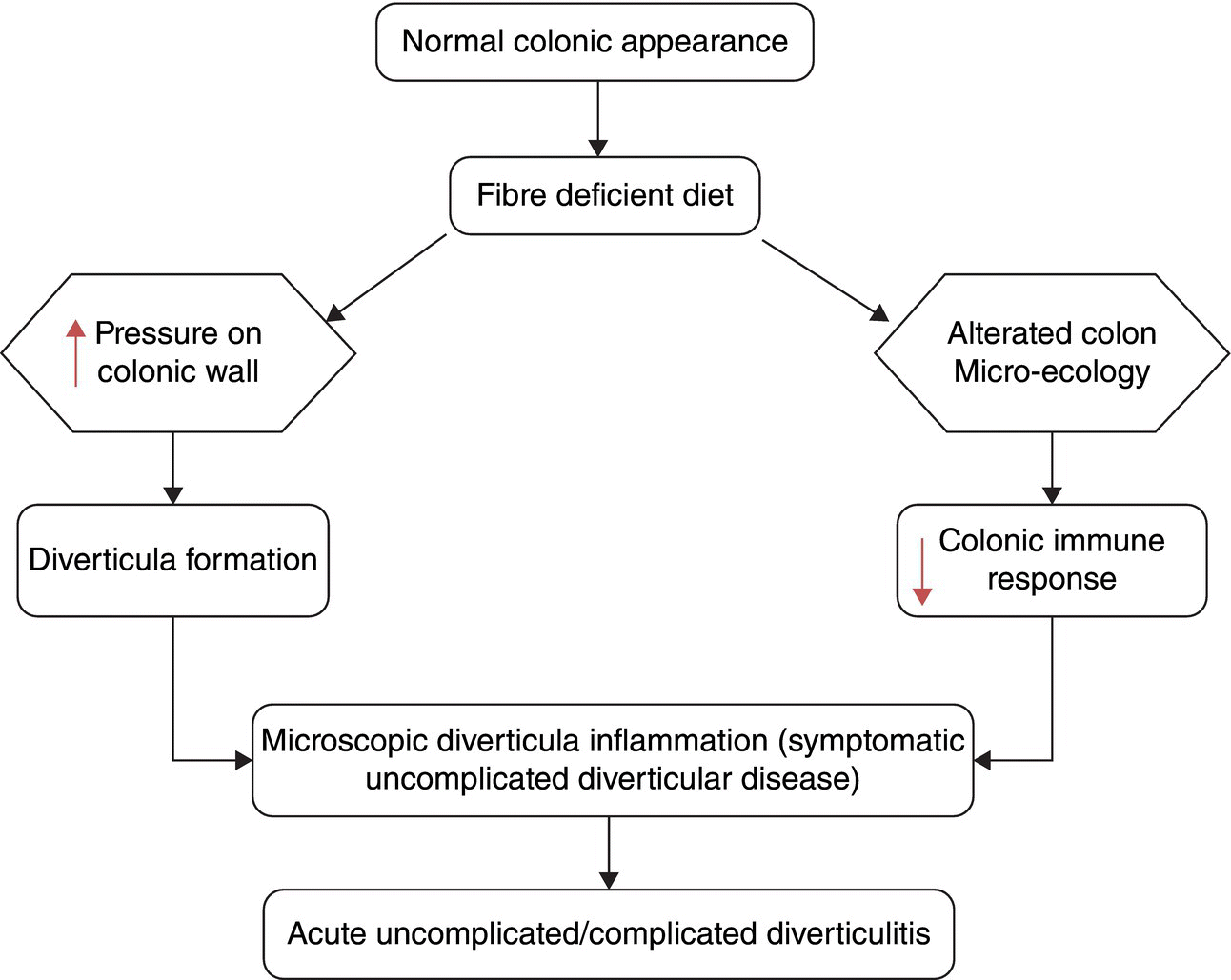
Figure 3.20.1 Pathogenetic events leading from diverticulosis to diverticular disease. Source: Tursi and Papagrigoriadis [4].
Table 3.20.1 Definition of terms in diverticular disease
| Term | Definition |
| Diverticular disease | The entire spectrum of asymptomatic to symptomatic disease associated with colonic diverticula |
| Diverticulosis | The presence of one diverticulum or, more commonly, multiple diverticula that are not inflamed |
| Diverticulitis | Diverticulosis with clinical symptoms and evidence of inflammation |
| Complicated diverticulitis | Disease state of diverticulitis including abscess, fistula, obstruction or free perforation |
Dietary fibre/non-starch polysaccharides
Dietary fibre is not a chemically defined material, unlike NSP, and will be used throughout this chapter (see Chapter 2.1 for definitions). Fibre is the part of fruit, grains or vegetables not digested in the GI tract and contributes to stool bulk. Restriction of fibre results in a reduction in the size and number of stools.
Lack of dietary fibre results in slower gut transit, greater water resorption and production of smaller firmer stools which are implicated in formation of diverticula. It is proposed that a low-fibre diet results in exaggerated contractions of the colonic circular muscle, dividing the lumen into a series of segments, raising the intracolonic pressure and leading to mucosal herniation [5,6]. Decreasing these effects is thought to decrease the likelihood of developing both diverticula and symptoms of DD [5,7].
When measurements are obtained from within the true sigmoid colon, there appears to be a link between exaggerated colonic motility, increased pressures and symptomatic DD [8,9] as well as abnormal motor and propulsive activities confined to the regions affected by DD [10]. However, there is considerable heterogeneity within these studies due to methodological factors that lead to scepticism about the link between altered colonic motility and DD and whether these findings play a role in pathogenesis or are simply related to diverticular symptoms.
Low-fibre diets and increased DD prevalence have also been shown to exist in non-Western populations in South Africa where the urban black population with higher dietary fibre intake (mean daily dietary fibre intake of 32.5 ± 11.4 g) than the local whites (mean daily dietary fibre intake of 22.4 ± 6.0 g) had an increased prevalence of DD compared to non-urban blacks with significantly higher dietary fibre intake [11]. It has to be noted, however, that this is a small population study and it is difficult to draw firm conclusions from it. In right-sided colonic DD commonly seen in the Asian populations of the Far East, similar increased risk is seen in urban populations with lower fibre intakes [12,13] with decreasing intake over time from 25.0 g/day per capita in 1946 to14.5 g/day in 1991 [14] coinciding with increased prevalence and daily dietary fibre intake of 17.4 ± 5.1 g in patients compared to 21.1 ± 6.6 g in controls [15].
Evidence exists that high-meat diets change bacterial metabolism in the colon [16]. It is possible that the interaction of red meat and bacteria results in production of a ‘toxic metabolite’ which promotes bowel wall spasm, resulting in weakening of the colon wall and diverticula formation [17]. Studies have revealed that a high intake of either red or processed meat correlates with a 2–4-fold increase in the risk of developing DD [18,19]. When compared to vegetarians, meat eaters were three times more likely to develop DD with diets containing half the dietary fibre of that in a vegetarian diet (21.4 g/day versus 41.6 g/day) [20]. A lifetime vegetarian diet, with a fibre intake of 42 g/day over 45 years, has been shown to be protective from DD compared to a meat eater’s diet of 21 g/day of fibre with a 20-fold difference in prevalence [21]. In a more recent UK study over a mean follow-up time of 11.6 years, vegetarians (22 g/day fibre intake in men and 21 g/day in women) had a 31% lower risk of DD compared with meat eaters (18 g/day fibre intake in men and women). The cumulative probability of admission to hospital or death from DD between the ages of 50 and 70 for meat eaters was 4.4% compared with 3.0% for vegetarians. Vegetarians also have faster colonic transit times and less disease, again probably due to the higher dietary fibre intakes [22].
A prospective study examining the relationship between red meat consumption and DD found age and energy-adjusted relative risk (RR) were significant for certain servings of meat, such as beef, pork and lamb as a main dish (RR 3.23); in sandwiches or mixed dishes (RR 1.98); processed meat (RR 1.90); bacon (RR 1.07); and hot dogs (RR 1.38). When further adjusted for dietary fibre intake and physical activity, consumption of red meat, as a single category, was still positively associated with risk of DD (RR 1.48) but did not reach statistical significance. Further analysis showed that the association of red meat with DD was not related to its protein or fat content. There was little association between DD and intake of chicken and fish or dairy fat [18].
The relationship between high-meat diets and DD extends to Asian patients as well, where the risk of right-sided disease may be increased by nearly 25 times compared with patients with low overall meat consumption [12].
Fat
A systematic review of dietary factors which were potential risk factors for the development of DD found that other than low fibre intake, high fat intake and high meat intake were associated [23]. Positive associations were found between DD and saturated, monounsaturated, trans-saturated and polyunsaturated fats, particularly in the presence of low fibre intake. A weak inverse association was observed for omega-3 fatty acids and DD. When adjusted for physical activity and dietary fibre, however, the association of DD with total fat and various types of fat was no longer significant [18].
3.20.2 Dietary effects of disease or its management
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






