Fig. 1
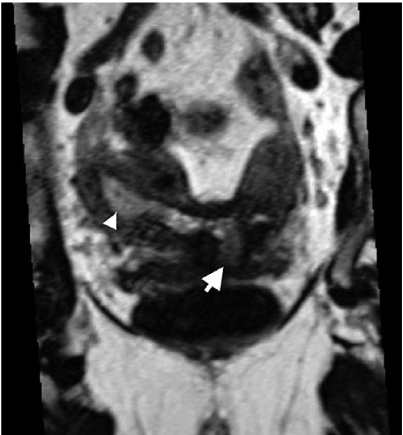
Normal magnetic resonance imaging (MRI) of the uterine anatomy on sagittal 3D T2-weighted multiplanar reconstruction (MPR) of the uterus and cervix demonstrates normal zonal anatomy. There are three zones identifiable in the uterus: hyperintense endometrium, hypointense junctional zone (arrow), and intermediate signal intensity of the outer myometrium. Four zones are distinguished in the cervix: hyperintense mucus within the endocervical canal, intermediate signal intensity of the cervical mucosa (arrowhead), hypointense cervical stroma, and intermediate signal intensity of the outer smooth muscle
Four zones are distinguished in the cervix by highresolution MRI:
Hyperintense mucus within the endocervical canal
Cervical mucosa (plicae palmatae) of intermediate to high SI
Hypointense cervical stroma surrounding the mucosa
Additional layer of intermediate SI in continuity with the uterine myometrium representing smooth muscle.
In postmenopausal patients, the uterine corpus but not the cervix regresses and decreases in size. On dynamic gadolinium-enhanced images, the endometrium is hypovascular relative to the myometrium and becomes iso- or hyperintense on delayed scans.
MRI Technique
If possible, patients should be scheduled in the second half of the menstrual cycle, as the thickness of the endometrial stripe increases during the follicular and secretory phase and thus the normal zonal anatomy of the uterus can be better appreciated. The objective of patient preparation is to obtain the best possible image quality while making the examination as comfortable as possible for the patient. To minimize motion artifact induced by bowel peristaltics, patients are advised to fast from 6 to 8 hours before the procedure. Unless contraindicated, intravenous or intramuscular injection of peristaltic inhibitors, i.e. glucagon or butyl-scopolamine, is recommended to further decrease artifacts. An empty urinary bladder minimizes ghosting artifact from patient motion. In addition, an empty bladder maintains the uterus in a more caudal position in the pelvis, away from small-bowel loops, and assures that the normally visible fat plane between the uterus and urinary bladder, an important criteria to exclude tumor invasion of the bladder wall in oncologic patients, will not be obliterated. Examinations are usually performed with the patient in the supine position using a body-flex phased array MR surface coil; in pregnant patients in the third trimester, imaging may also be performed in an oblique or lateral-decubitus position.
Depending on the clinical questions, one or several fast sequences of the upper abdomen should be performed, e.g., cine steady-state free-precession sequence [true fast imaging with steady-state precession (True-FISP); fastimaging with steady-state acquisition (FIESTA)] in the coronal and axial plane and a T1-weighted (T1-W) gradient echo (GRE) sequence, to exclude ascites, enlarged retroperitoneal lymph nodes, hydronephrosis, or renal agenesis in patients with congenital uterine malformations.
T1-W sequences are standard for assessing the small pelvis. Although uncommon, lipomatous tumors of the uterus can only be accurately diagnosed with an additional fat-suppressed T1-W sequence using chemical presaturation to distinguish fat from blood, mucin, or other proteinaceous material, which remain of high SI. Alternatively, a T1-W 3D Dixon GRE sequence allows for T1- W in-phase, T1-W out-of-phase, T1-W water excitation, and T1-W fat excitation images within a single breathhold. Routine use of this sequence helps standardize imaging protocols without additional exam time.
T2-W images are most important for assessing the uterus, whereas sagittal sections are best suited to image it. Oblique coronal and axial slice orientation according to the long and short axis of the endometrial cavity should be applied for assessing uterine and cervical pathologies. It is important to train MR technologists on how to acquire these oblique sequences if physician supervision is not possible. The short-axis coronal oblique sequence (perpendicular to the long-axis of the endometrial cavity) is particularly valuable for assessing localized endometrial pathology and evaluating junctional zone thickness. It is also valuable for determining the extent of a uterine septum. A T2-W sequence performed parallel to the long axis of the endometrial cavity is critical to characterize the external uterine contour in patients with Müllerian duct anomalies. As this series is so important for classifying uterine anomalies, it is best performed earlier in the examination, prior to bladder filling that often displaces the uterus as it becomes increasingly distended. If the fundal contour is inadequately characterized by T2-W images, T1-W images parallel to the long axis can help characterize the external contour due to increased contrast between the myometrial fundal contour and the overlying fat. Software advances allow for 3D T2-W acquisition, facilitating postprocessing of the uterus in any plane and potentially obviating the need to acquire individual orthogonal 2D T2-W sequences. However, there is a trade off between acquisition time and left-to-right coverage, which is the result of trying to acquire nearly isotropic voxels for optimum multiplanar reconstruction.
Imaging of the uterus should be performed using the smallest possible field of view (FOV) (20–24 cm), thin sections (4–5 mm), and the largest possible matrix size appropriate to each individual sequence. These imaging parameters provide important anatomic detail, which becomes critical when imaging uterine anomalies and endometrial pathology. When imaging large leiomyomas, section thickness and FOV may need to be adjusted accordingly. However, when establishing the myometrial origin of a subserosal leiomyoma, thin sections at the level of the pedicle are frequently helpful.
We added an axial echoplanar diffusion-weighted (DW) MRI sequence of the small pelvis to our clinical routine protocol. DWI, well established for intracranial imaging, is a functional technique in which contrast derives from the random motion of water molecules within the extracellular tissue. It has a potential for identifying pelvic tumors, i.e., to differentiate benign from malignant lesions, and has a high sensitivity to detect iliac lymph nodes.
Gadolinium-enhanced imaging is not needed for most benign conditions but can be useful in selected pathologies. We routinely perform contrast-enhanced imaging in patients referred for evaluation of endometrial pathology. In addition, contrast-enhanced imaging is performed in patients with symptomatic leiomyomas scheduled for laparoscopic surgery or uterine artery embolization, in the latter case with additional MR angiography (MRA) of the iliac arteries. Vascularity can help predict treatment response, and enhancement may help distinguish a benign fibroid from a malignant leiomyosarcoma.
Congenital Uterine Anomalies
Between the 6th and 11th weeks of gestation, the paired Müllerian ducts undergo descent, fusion, and septum resorption to form the uterus, fallopian tubes, cervix, and upper two thirds of the vagina [2]. Müllerian duct anomalies (MDA) occur when this process either fails or is interrupted. Depending on the anomaly, some patients may be asymptomatic, with normal fertility and obstetric outcomes, whereas others may have primary amenorrhea, menstrual irregularities, infertility, obstetric complications, and/or endometriosis. The reported prevalence of MDA varies widely: 1–5% in the general population and 13–25% in women with recurrent fetal loss [3]. Ovaries and external genitalia/distal one third of the vagina are spared because they originate from the primitive yolk sac and sinovaginal bud, respectively; renal anomalies are present in 30–50% of women with MDA [4].
Buttram and Gibbons classified MDAs in 1979, and a revised classification by the American Society for Reproductive Medicine (formerly the American Fertility Society) was published in 1988 [5, 6] (Fig. 2); appropriate classification is critical to ensure proper treatment. Whereas most anomalies can be assigned a subtype, some defy specific classification and are best described anatomically rather than “forced” into a category.
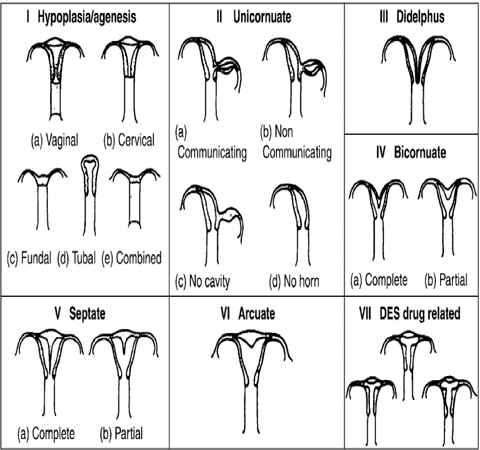

Fig.
Classification system of Müllerian duct anomalies developed by the American Fertility Society [6]
When an MDA is suspected, US is the most commonly performed exam and may be diagnostic, especially if a 3D US is performed. However, MRI remains the preferred imaging technique in patients where EVUS is not possible due to vaginal agenesis, in patients with complex uterine anomalies, and especially when surgical intervention is contemplated. In multiple studies, MRI shows excellent agreement with MDA subtype classifications [7].
Class I: Müllerian Agenesis and Hypoplasia
Müllerian agenesis and hypoplasia anomalies (10%) occur early in gestation and are the result of some degree of failure of the Müllerian ducts prior to fusion [2]. The most extreme and common form of class I MDA is the Mayer- Rokitansky-Küster-Hauser syndrome: agenesis of the vagina, cervix, and uterus in 90% of cases. In 10%, there is a rudimentary uterus present. Ovaries are morphologically and biochemically normal, but they may be located outside the pelvis. Treatment is focused on enabling sexual function by creating a neovagina. At imaging, fatty and connective tissue appears in the expected location of the upper two thirds of the vagina and is best seen on transverse images (Fig. 3). A truncated vagina may be seen between the urethra anteriorly and the rectum posteriorly.
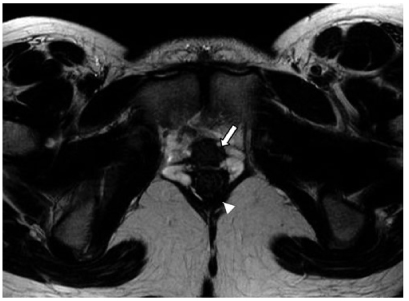

Fig. 3
Müllerian duct anomalies (MDA): Mayer-Rokitansky-Küster- Hauser Syndrome. Axial T2-weighted magnetic resonance image (MRI) in a woman with primary amenorrhea demonstrates the upper urethra anteriorly (arrow) and rectum posteriorly (arrowhead). A normal upper vagina is not present. The transverse plane highlights the fibrofatty tissue in the expected location of the vagina
Class II: Unicornuate Uterus
Unicornuate uterus (20%) is the result of normal development of one Müllerian duct, with either complete agenesis or some degree of arrested development of the contralateral duct. There are four subtypes of unicornuate uterus:
No rudimentary horn
Rudimentary horn without a cavity
Rudimentary horn with communicating cavity
Rudimentary horn with noncommunicating cavity.
There is a high association with renal anomalies, especially renal agenesis, ipsilateral to the rudimentary horn (40%). Only patients with cavitary rudimentary horns require surgical excision. This is done to prevent hematometra and/or obstetric complications to include uterine rupture [8].
At imaging, the unicornuate uterus is deviated to one side of the pelvis, and the endometrium is described as “banana” or “bullet” shaped (Fig. 4). Uterine zonal anatomy is preserved, as is the endometrial-to-myometrial ratio [9]. Appearance of the rudimentary horn varies by subtype. A noncavitary rudimentary horn images as diffuse low to intermediate SI. A cavitary rudimentary horn displays normal zonal anatomy. If noncommunicating, the endometrial cavity may be distended with blood, and/or there may be imaging manifestations of endometriosis.
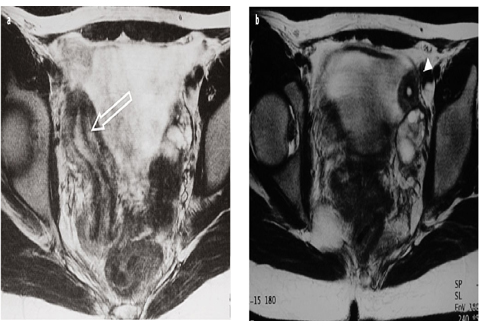

Fig. 4 a, b
a Müllerian duct anomalies (MDA): unicornuate uterus. Axial T2-weighted magnetic resonance images (MRI) show: a right-sided “banana”-shaped unicornuate uterus (open arrow) and b smaller, noncommunicating cavitary horn (arrowhead)
Class III: Uterus Didelphys
Uterus didelphys (5%) results from complete failure of Müllerian duct fusion. Most patients have two distinct uterine horns, cervices, and upper vaginas [3]. A transverse hemivaginal septum is often present, with ipsilateral obstruction and hematometrocolpos. If present, this septum is excised. Patients with obstructed uterus didelphys are more likely than others to have ipsilateral renal agenesis [4]. Patients without a transverse hemivaginal septum are asymptomatic and require no invasive therapy.
At imaging, two widely divergent and symmetric uterine horns, two separate cervices (“owl eyes”), and two separate upper vaginas are noted (Fig. 5). There is no communication between the paired endometrial or endocervical cavities. A fundal cleft >1 cm is always present [10]. Uterine zonal anatomy and endometrial-to-myometrial ratio are preserved in both horns. If there is an obstructing transverse hemivaginal septum, the affected side will be distended, with high SI blood products on T1-W sequences.
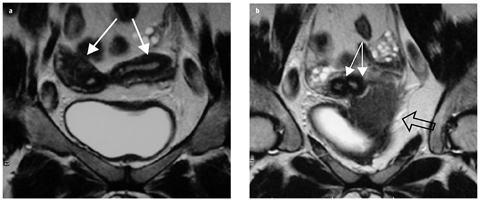

Fig. 5 a, b
Müllerian duct anomalies (MDA): uterus didelphys. Coronal T2-weighted magnetic resonance images (MRI) demonstrate: a two divergent uterine horns (arrows) and b separate cervices (solid arrows). There is no communication between the paired cavities. A transverse left hemivaginal septum obstructs the ipsilateral vagina, leading to left hematocolpos (open arrow, b)
Class IV: Bicornuate Uterus
Bicornuate uterus (10%) results from incomplete fusion of the Müllerian ducts. The uterine horns are duplicated, and there may be a single (bicornuate unicollis) or a duplicated (bicornuate bicollis) cervix. Communication of the endometrial or endocervical canals is necessary to make the diagnosis. In one fourth of cases, there will also be a longitudinal vaginal septum, and distinction with uterus didelphys can be challenging. In the absence of symptoms, no treatment is necessary. If a patient is symptomatic (dyspareunia and/or hematocolpos), a vaginal septoplasty is warranted [11, 12].
Imaging features include two symmetric uterine horns separated by a deep (>1 cm) fundal cleft (Fig. 6). In a bicornuate unicollis uterus, the endometrial canal is heart shaped, with visible communication between the two sides. In a bicornuate bicollis uterus, the two cervices mimic “owl eyes” of an uterus didelphys. Communication between segments allows diagnosing bicornuate bicollis with confidence.