Chapter 37 Diagnostic Cholangiography
Introduction
Endoscopic cannulation of the major papilla with imaging of the biliary tree and the pancreatic ductal system (endoscopic retrograde cholangiopancreatography [ERCP]) was first successfully accomplished with an end-viewing duodenoscope and reported in 1968.1 Subsequent development of side-viewing endoscopes with a catheter-deflecting elevator greatly facilitated the technique. Diagnostic studies were supplemented by the first endoscopic sphincterotomies in the early 1970s.2,3 These developments permitted less invasive diagnostic and therapeutic maneuvers in the bile duct previously limited to open surgical and percutaneous techniques. Although these procedures are more technically demanding than most other gastrointestinal (GI) endoscopic techniques, they are now being widely used and are the method of choice for many clinical problems involving the pancreatic ductal and hepatobiliary systems.
This chapter focuses on diagnostic endoscopic retrograde cholangiography (ERC). Radiographic visualization of the biliary tree is often key to establishing a clinical diagnosis and formulating a therapeutic plan.4,5 With the aid of noninvasive imaging via transcutaneous ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI),6–13 thorough ductal filling at ERC is needed less frequently and is contraindicated in certain cases. Diagnostic ERC is just one portion of commonly combined ERCP and associated therapeutic maneuvers.
Endoscopic Retrograde Cholangiopancreatography
Indications and Contraindications
The role for diagnostic ERC alone has nearly disappeared as other, less invasive and noninvasive imaging techniques (e.g., CT scans, endoscopic ultrasound [EUS], magnetic resonance cholangiopancreatography [MRCP]) have become more widely used. Imaging of the biliary ductal system without anticipated therapy is clinically helpful in only a few clinical settings, such as cholestasis without dilated ducts. In certain settings, such as inflammatory bowel disease, patients with early sclerosing cholangitis may have ductal changes visible only via invasive cholangiography such as ERC (i.e., missed by noninvasive imaging). ERC is mainly indicated in clinical settings in which there is significant suspicion of obstructing, inflammatory, or neoplastic pancreatobiliary lesions that, if detected or ruled out, would alter clinical management. A general classification of indications is listed in Box 37.1.
Most contraindications of ERC are relative, and the degree of risk must be balanced against the potential benefit.14–16 In certain settings, even very ill and unstable patients, such as with acute cholangitis with shock or sepsis from bile duct stones or biliary strictures, diagnostic (followed by therapeutic) ERCP may be lifesaving. ERC in patients with necrotizing acute pancreatitis and low clinical suspicion for ductal stones is considered relatively contraindicated because pancreatography may result in bacterial contamination of the pancreatic bed. Other relative contraindications include unstable cardiopulmonary disease or severe coagulopathy. Patients with comorbid life-threatening conditions can often have ERC performed in the intensive care unit (with or without fluoroscopy) if deemed medically necessary. ERC is generally not indicated in type III suspected sphincter of Oddi dysfunction (unless manometry is included).
Preparation for Endoscopic Retrograde Cholangiography
Assembling the Team
We recommend that ERCP be done independently only by physicians with prior formal training in ERCP. An adequate number of examinations during training varies greatly with the trainee but should include at least 200 examinations.17 This number includes at least 100 therapeutic examinations. Successful biliary cannulation rates should be at least 85% and preferably 90%. We recommend that nurses have experience with at least 1000 upper GI and colonoscopy examinations before “graduating up” to the ERCP suite. Nurses should train alongside experienced ERCP nurses for 100 to 200 examinations before independent guidewire and accessory management is undertaken. Two nurses are needed per examination: one for sedation and analgesia administration and one for accessories management. Radiology technicians working in ERCP should maintain longevity and be team members rather than rotate frequently. In nearly all centers, a radiologist no longer assists in fluoroscopy or image acquisition except in the most difficult cases. Collaborative reading of final images may aid in the accuracy of final interpretation. Final reading by general radiologists with little pancreatobiliary training, experience, or interest may be counterproductive.
Patient Preparation
Risk factors such as anticoagulant therapy, prosthetic heart valves, and allergies must be addressed.18 Patients with iodine allergy are at very low risk of allergic reaction19; nevertheless, some centers continue to use prednisone, 30 to 40 mg orally, 15 hours and 3 hours before the examination. Diphenhydramine (Benadryl), 25 mg intravenously, may be added if serious past reactions have occurred. Iodine allergy is not a reason to omit a needed examination, but limiting the volume of contrast medium used is logical. Air20 can be successfully used for cholangiography if needed. If possible, aspirin and nonsteroidal antiinflammatory drugs should be avoided for 7 days before the procedure.
Informed Consent
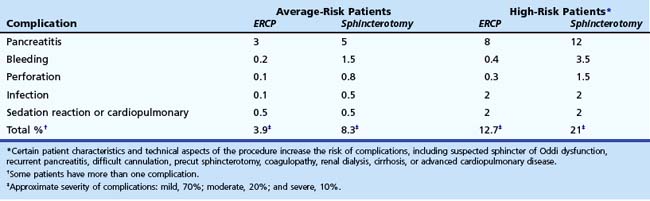
Informed consent for ERCP must be obtained. It is both legally and ethically necessary to apprise the patient (and family members if applicable) of the risks, benefits, and alternatives of the anticipated procedure. Table 37.1 lists potential complications of diagnostic and therapeutic ERCP and their relative frequency. Although legal standards continue to evolve, we recommend that patients be informed of the potential complications and the relative frequencies of complications. In addition, we recommend that patients be told that a severe complication possibly may result in a prolonged hospital stay, intensive care unit monitoring, or open surgery and very rarely may result in permanent disability or death. Complication rates vary according to patient and procedure risk factors and the disease process being evaluated and treated. Patients with uncomplicated biliary stones, malignancy, or chronic pancreatitis have lower complication rates, whereas patients with acute recurrent pancreatitis and suspected sphincter of Oddi dysfunction have twofold to fourfold higher complication rates. Procedure techniques associated with higher complication rates include repeated cannulation attempts, repeated pancreatic duct injections, pancreatic parenchymal acinarization, and precut sphincterotomy (without associated protective pancreatic stent). Attention to details of the technique and patient selection can minimize, but not eliminate, complications. Morbidity can be limited by early recognition and treatment of complications.
Endoscopic Equipment
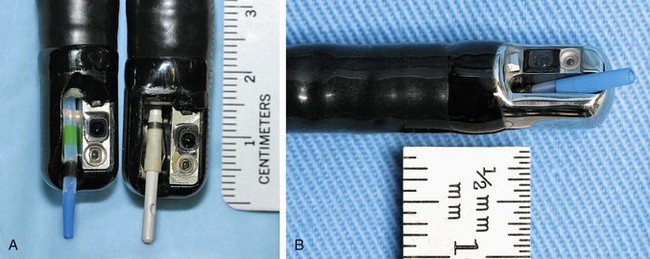
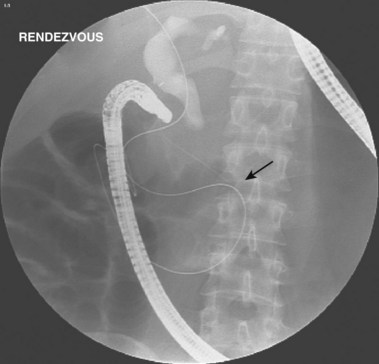
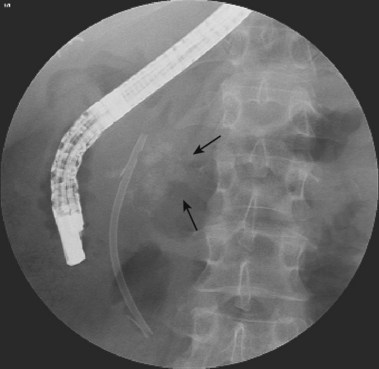
ERC can be performed with fiberoptic or video chip, side-viewing instruments. Endoscopes are 120-cm working length and generally categorized as diagnostic (approximately 10-mm diameter) or therapeutic (12- to 13-mm diameter). Video systems offer the advantage of television monitor viewing by all persons in the endoscopy suite; this offers better teaching capabilities and allows better coordination between the endoscopist and nursing assistants. Some newer generation endoscopes combine a large working channel diameter up to 4 mm with a standard 10- to 11-mm outer diameter (Fig. 37.1). A newer generation pediatric videoendoscope with outer diameter less than 7 mm is now available from Olympus America Inc. Most ERCP examinations are done with air insufflation. Limited data show that carbon dioxide inflation reduces postprocedure abdominal distention.21 For patients undergoing Billroth II procedures, we generally start with a standard side-viewing duodenoscope, but an end-viewing endoscope is occasionally needed. In patients with a long Roux-en-Y gastroenterostomy or choledochojejunostomy, a 160-cm pediatric colonoscope or a 220-cm enteroscope can reach the bile duct in greater than half of patients.22 Double-balloon enteroscopy has facilitated Roux-en-Y limb traversal.23,24 The lack of a catheter-deflecting elevator and limited compatibility accessories make end-viewing endoscopy difficult in these settings. Rendezvous with transhepatic wire passage is occasionally helpful (Fig. 37.2).
Current-generation endoscopes are capable of undergoing submersion disinfection. After cleaning, endoscopes should be hung in vertical position to facilitate drying. In the past, Pseudomonas infections were directly linked to inadequate ERCP scope disinfection. Ideally, endoscopes should be cultured periodically. Patients developing infections after undergoing ERCP should be cultured for the presence of Pseudomonas species (see Chapter 4).
Radiology Suite
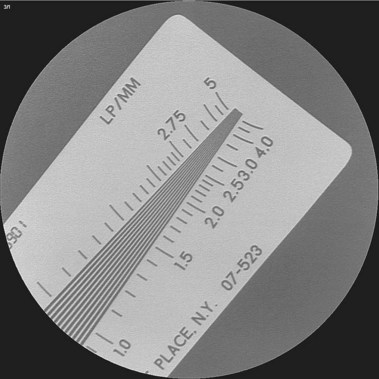
Few endoscopists have a dedicated suite for ERCP. We are aware of no manufacturer who markets a fluoroscopy unit specifically for ERCP. Most endoscopists schedule time in the radiology department and use general purpose or angiographic units. Film documentation is likely to disappear in the next decade, with digital formats viewed only on high-resolution monitors, which are becoming the new standard. Quality digital images now rival film quality. Flat tables with fixed overhead carriage have limited versatility. The preferred x-ray table includes the capability to tilt the patient’s head up and down 30 degrees and has C-arm carriage, which allows axial, cranial, caudal, vertical, and horizontal movements, allowing viewing at multiple angles (Fig. 37.3). Because the patient is usually positioned prone with the head at the “foot” of the table, ability to reverse the viewing image in both the vertical and the horizontal axes is helpful. In the past, endoscopists used older generation x-ray units, including portable C-arm units with limited image resolution. This practice is no longer acceptable because fluoroscopy and saved image quality are key to accurate diagnosis and management. High-quality ERCP imaging requires resolution equivalent to that for neuroradiology (brain blood vessels). Resolution of greater than 2.5 line pair per millimeter is strongly recommended for both fluoroscopy and final images (Fig. 37.4). This resolution is best accomplished with smaller diameter image intensifiers of 6 to 9 inches.
Radiation safety standards should be followed.25 Monitoring of personal exposure and review of methods to limit exposure are needed. Attention to coning the field of view to the area of interest is good practice. Lead aprons or shields around the patient limit x-ray beam scatter. Use of newer generation pulse fluoroscopy gives intermittent viewing, which is slightly jerky but often adequate with one-tenth the radiation exposure. Appropriate lead aprons, lead glasses, and thyroid shields are recommended (Fig. 37.5).
Technique
Patient Positioning, Preparation, and Sedation
Most centers prefer to have the patient positioned in a prone or slightly left lateral decubitus position on a fluoroscopic table. Less often, supine position is preferred, such as in patients with recent abdominal incisions, patients with multiple abdominal drain tubes, and patients undergoing Billroth II procedures.26 Intravenous access and monitoring equipment for blood pressure, pulse, and pulse oximetry are needed. Electrocardiogram (ECG) monitoring is desirable for patients with angina or a history of a cardiac arrhythmia and in other less stable patients.
Sedation and analgesia are achieved by slow intravenous administration of diazepam (10 to 40 mg), midazolam (2 to 10 mg), and meperidine (25 to 150 mg) or fentanyl (50 to 150 mg). The general ranges mentioned here are given over a 30- to 60-minute examination. Droperidol (2.5 to 10 mg) is a common supplement or alternative, particularly for alcoholics or persons regularly taking narcotics or benzodiazepines.27 However, more recent concerns about arrhythmias and Q–T interval prolongation have limited the use of droperidol for endoscopy. Our policy is to use droperidol routinely for patients whose baseline ECG has a normal corrected Q–T interval.28 More recently, propofol has been used for deep sedation and may offer better procedure tolerance and a much shorter recovery time than standard sedation. Propofol use, when administered by endoscopists and endoscopy nurses, is apparently safe for standard upper GI endoscopy and colonoscopy but has not been well studied for ERC.29–31
Upper Gastrointestinal Endoscopy
Before attempts at cannulation, fluoroscopic visualization (or still image acquisition) of the field of interest should be performed to look for stents, calcifications, masses, and residual contrast material (Fig. 37.6). The choice of initial cannulation tool is a personal preference (similar to choosing a tennis racket or golf club). One may begin with a simple single-lumen 5-Fr polyethylene catheter, without a guidewire, in many cases. The relative flexibility (not rigid), maneuverability, low cost, and simplicity are attractive features. A manometry catheter is initially used if ERCP findings are likely to be nonspecific or normal and if manometry is likely to be potentially helpful. If a sphincterotomy is almost certainly needed, a sphincterotome is a good starting tool. If the orifice appears small, a more tapered tip catheter or sphincterotome may be chosen. A few centers prefer various shaped metal tip catheters. Two-lumen or three-lumen catheters or sphincterotomes may be preferred because they have a separate lumen for a guidewire and contrast medium. A guidewire may be used at any point to aid cannulation or maintain intraductal stability. For biliary cannulation, 0.025-inch or 0.035-inch diameter wires are preferred. Soft-tipped wires have the advantage of less tissue trauma (e.g., fewer submucosal or other extraductal dissections). The specific devices used are much less important than the skill of the endoscopist (Fig. 37.7).
If the major papilla is not initially evident, gentle lifting of folds, greater air distention, and use of glucagon to inhibit peristalsis would likely expose the structure. If duodenal diverticula are present, the major papilla is most commonly on the diverticular rim, but the papilla is within the diverticulum per se in approximately 5% to 10% of cases (Fig. 37.8). The major papilla is then cannulated. Orientation of the catheter tip toward the 11 o’clock to 12 o’clock position (Fig. 37.9) would more likely enter the bile duct; orientation of the catheter toward the 3 o’clock to 5 o’clock position would more likely enter the pancreatic duct. Biliary orifice location may vary from 10 o’clock to 2 o’clock. Cannulation may be initially done by gentle impaction of the catheter tip in the papillary orifice. Deep cannulation (>1 cm penetration of the catheter into the duct) more securely establishes an intraductal position, which allows contrast agent injection, fluid aspiration, patient position changes, and endoscope position changes without loss of access to the duct.
Selective Deep Biliary Cannulation
Pancreatic cannulation is easier than biliary cannulation. Selective biliary entry is mandatory in most cases of biliary pathology. We often start with a standard 5-Fr catheter and add a guidewire or a sphincterotome for assistance. If the cannulation angle fails to achieve adequate cephalad orientation, a sphincterotome or curved top guidewire generally helps to achieve that angle. There are increasing observations that initial use of a guidewire facilitates cannulation and decreases post-ERCP pancreatitis.32
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree