Incidental small renal masses identified on imaging are increasingly investigated via needle core or fine needle aspiration biopsies with limited material provided for rendering a diagnosis. Lesions with a prominent eosinophilic or oncocytic cell presence showing morphologic overlap between well-known eosinophilic neoplasms are challenging to diagnose. We review the range of known benign and malignant eosinophilic renal neoplasms and their immunoprofiles to elucidate a useful panel of stains that may assist the pathologist in making an accurate diagnosis.
Key points
- •
CK7, S100A1, vimentin/c-KIT, and Claudin 7/8 can help to differentiate renal oncocytoma and chromophobe renal cell carcinoma (RCC).
- •
CAIX, CK7, racemase, CD117, and CD10 can help to differentiate oncocytoma, chromophobe RCC, clear cell RCC, and papillary RCC.
- •
“High-grade” nuclear features are seen in renal neoplasms with wide-ranging clinical behavior. Immunomarkers are useful in differentiating these entities.
- •
Unique markers and molecular tests are helpful to diagnose certain neoplasms, such as translocation-associated RCC and hereditary leiomyomatosis-related RCC.
Introduction
Immunohistochemical biomarkers are useful when diagnosing renal cell carcinomas (RCC) with less than straightforward morphology or for confirming the presence of metastatic carcinoma of renal origin. They have been proven to increase the accuracy of diagnosis in limited biopsy material. Incidental small renal masses identified on imaging are increasingly investigated via needle core or fine needle aspiration biopsies with limited material provided for rendering a diagnosis. These lesions are amenable to treatment by noninvasive techniques, such as ablation, instead of resection. Consequently, the readily available immunohistochemical stains take on a more significant role in current practice.
RCC with distinct morphologies do not pose much difficulty in daily diagnostic practice. Moreover, many of these have well-described immunoprofiles ( Fig. 1 , Table 1 ). The challenge lies in lesions with a prominent eosinophilic or oncocytic cell presence and where there is morphologic overlap between the well-known eosinophilic neoplasms. Additionally, with only limited biopsy material, the onus is on the pathologist to rule out an eosinophilic neoplasm with potentially aggressive behavior without the reassurance of subsequent confirmation by nephrectomy. The impact on patient care of missing such a diagnosis is not inconsequential. As such, we review the range of known benign and aggressive eosinophilic renal neoplasms and their immunoprofiles to elucidate a useful panel of stains that could be used in this scenario.
| Renal Tumors | Positive Markers | Negative Markers |
|---|---|---|
| Clear cell RCC | Vimentin, keratin, EMA, CD10, RCCm, Pax2/8, CAIX | CK7, ksp-cadherin, parvalbumin |
| Papillary RCC | Keratin, CK7, AMACR, RCCm | c-KIT/CD117, ksp-cadherin, parvalbumin, WT-1 |
| Chromophobe RCC | e-Cadherin, ksp-cadherin, c-KIT/CD117, EMA, CK, CK7 | Vimentin, CAIX, AMACR |
| Oncocytoma | ksp-Cadherin, c-kit/CD117, parvalbumin, S100A1 | CK7, moc31, EP-CAM |
| Translocation RCC | TFE3/TFEB, CD10, RCCm | CK |
| Collecting duct RCC | EMA, p63, CK7, HMWCK, Pax2/8 | CD10, RCCm, CK20 |
| Angiomyolipoma | HMB45, Melan-A, SMA | CK, CD10, RCCm, Pax2/8 |
| Tumors with papillary architecture | ||
| Papillary RCC | Type 1: CK7 | CK20, 34BE12, ULEX-1, Type2: CK7 |
| Collecting duct carcinoma | CK7, CK20(focal+), 34BE12, ULEX-1 | CK20 |
| Urothelial carcinoma | CK7, CK20, 34BE12, ULEX-1 | — |
Renal Oncocytoma Versus Chromophobe Renal Cell Carcinoma
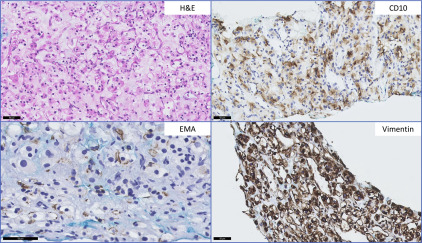
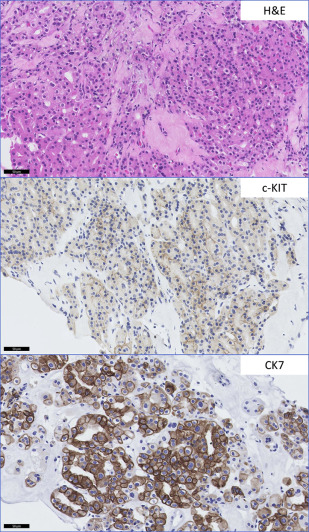
The most commonly encountered diagnostic dilemma of a low-grade nonpapillary oncocytic renal neoplasm is between renal oncocytoma and a chromophobe RCC (ChRCC), eosinophilic type. Morphologic heterogeneity can be seen in ChRCC with foci exhibiting features virtually indistinguishable from oncocytoma. Although the majority of ChRCC are regarded to have favorable prognosis, a small subset of patients show disease progression. Many well-established and novel biomarkers have been tested for use in this context. However, few have been validated in more than 1 series. CK7 positivity in ChRCC is well-described and considered to be useful in differentiating ChRCC from benign renal oncocytoma, although some studies have shown similar expression in both entities ( Fig. 2 ). Liu and colleagues, in determining a practical panel to distinguish clear cell RCC (CCRCC), ChRCC, and oncocytoma, confirmed the usefulness of CK7 and additionally found that Ep-CAM identified 100% of ChRCC with membranous or basolateral staining whereas only 29% of oncocytomas showed focal positive staining. A similar study confirmed Ep-CAM and CK7 staining in ChRCCs (80%) with no staining seen in oncocytomas. Claudin 7, a distal nephron marker identified by gene expression microarray analysis, is another candidate marker recommended for use as part of a panel, identifying 76% of ChRCC and 26% of oncocytomas in immunohistochemical validation studies. A further study showed the combination of Claudin 7 (membranous or mixed membranous/cytoplasmic stain positivity) and Claudin 8 (negativity) reliably differentiated ChRCC from oncocytomas (Claudin 7-negative/Claudin 8-positive with membranous, mixed membranous/cytoplasmic or perinuclear staining). Osunkoya and colleagues also showed the combination of Claudin 7-positive (membranous)/Claudin 8-negative profile had an 88% positive predictive value for ChRCC whereas Claudin 7-negative/Claudin 8-cytoplasmic staining had a 100% specificity and positive predictive value for oncocytoma. S100A1, a transduction protein involved in cell-cycle progression, was reported to be expressed in 93% of oncocytomas and not expressed by ChRCC. It was 1 of 4 candidate proteins subsequently reported by Carvalho and colleagues to be useful in discriminating oncocytoma from its mimics—ChRCC, papillary RCC (PRCC), and CCRCC—using hierarchical and supervised cluster analyses. S100A1-positive/CK7-focal positive distinguished oncocytoma from ChRCC with 91% sensitivity and 93% specificity. The authors also found the combination of vimentin-negative/c-KIT–positive distinguished oncocytoma from CCRCC with 83% sensitivity and 86% specificity, and from PRCC with 79% sensitivity and 88% specificity ( Table 2 ).
| Immunomarker | Oncocytoma | ChRCC | CCRCC | Translocation RCC | PRCC | Follicular Thyroidlike RCC | RCC, Unclass (“Low grade”) | RCC, Unclass (“High grade”) | eAML | HLRCC | SDH Mutation RCC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TFE3 | TFEB | |||||||||||
| Unique marker | — | — | — | TFE3+ | TFEB+ | — | TTF-1 − TG − | — | — | — | FH mutation | SDHA − SDHB − |
| CK7 | − (Focal +) | + | − | − | — | Type 1 + Type 2 − | Mostly − | + | — | — | − (Focal +) | − |
| Ep-CAM | Focal + | + | — | — | — | — | — | — | — | — | — | — |
| Claudin 7/8 | −/+ | +/− | — | — | — | — | — | — | — | — | — | — |
| S100A1 | + | − | + | — | — | + | — | — | — | — | — | — |
| Vimentin/c-KIT | − (Mem +)/+ | −/+ | +/− | — | — | +/− | − | — | — | — | — | — |
| EMA | — | — | + | — | − | — | − | — | + | − | — | — |
| AE1/AE3 | — | — | + | — | − | — | + | — | + | − | — | — |
| Vimentin | — | — | + | — | — | — | — | — | — | — | — | — |
| PAX 8 | — | — | + | — | — | — | — | — | + | − | — | — |
| CAIX | — | − | Mem + | — | — | + | — | — | — | — | — | — |
| RCCm | — | − | + | + | − | + | − | — | — | — | — | — |
| CD10 | — | − | Mem + | + | − | Lum + | − | — | — | — | — | — |
| AMACR | — | — | — | + | — | + | − | — | — | — | — | — |
| e-Cadherin | — | + | − | + | — | — | — | — | — | — | — | — |
| MelanA/HMB45 | — | — | — | — | + | — | — | — | — | + | — | — |
In a recent report, S100A1 combined with HNF1b showed discriminatory potential where 73% of oncocytomas and 21% of ChRCC were nuclear positive for HNF1b and 80% of oncocytomas and 8% of ChRCC were positive for S100A1. No ChRCC were positive for both markers. However, these results are yet to be validated.
Clear Cell Renal Cell Carcinoma with Eosinophilic Morphology Versus Translocation-Associated Renal Cell Carcinoma
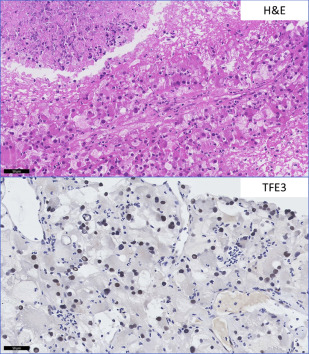
CCRCC with a predominant eosinophilic morphology is commonly seen in high-grade tumors with accompanying hemorrhage and necrosis, but is not considered a separate entity because it shares molecular characteristics with conventional CCRCC with the classic clear cell morphology. The translocation-associated RCCs, characterized and defined by translocations involving MiTF/TFE family genes, may share high nuclear grade morphology and granular eosinophilic cytoplasm. When other characteristic features such as papillary architecture and psammoma bodies are absent, ancillary studies are helpful. Conventional RCC is positive for CD10, CAIX, EMA, vimentin, and negative for CK7 and high-molecular-weight cytokeratin. MiTF RCC is negative for AE1/AE3 and EMA, and negative or focally positive for vimentin. TFE3 carcinomas are positive for RCCm, CD10, AMACR, and e-cadherin. TFEB tumors are CD10 and RCCm negative or focally positive, and positive for the melanocytic markers, Melan-A and HMB-45 (rarely expressed in TFE3 tumors) (see Table 2 ). Unique TFE3 and TFEB immunomarkers are highly sensitive and specific for these tumors in appropriately fixed tissue but break-apart fluorescence in situ hybridization is used for a definitive diagnosis ( Fig. 3 ).
Renal Cell Carcinoma, Unclassified (“Low-Grade Oncocytic Type”) Versus Oncocytoma and Chromophobe Renal Cell Carcinoma, Eosinophilic Type
An RCC is “unclassified” when morphologic features do not fit into any recognized class, or 2 or more morphologic types, or purely sarcomatoid carcinoma is present. These are usually high-grade carcinomas. However, a subset of these tumors have “low-grade” features and include an oncocytic-type characterized by solid nests or alveoli with oncocytoma-like architecture, nuclear pleomorphism and mitotic index beyond that which is acceptable for oncocytoma. No nuclear wrinkling or perinuclear halos, as seen in the eosinophilic ChRCC are present; features that may be absent in a limited biopsy sample. “Low-grade” unclassified RCC are diffusely positive for CK7, which definitively rules out oncocytoma, but does not exclude ChRCC.
Follicular Thyroid-like Carcinoma Versus Papillary Renal Cell Carcinoma and Renal Cell Carcinoma, Unclassified (“Low-Grade Oncocytic Type”)
A recently described and little-studied entity, follicular thyroid-like carcinoma, closely mimics well-differentiated thyroid follicular neoplasms, recapitulating the microfollicular and macrofollicular architecture, lined by amphophilic to eosinophilic cells with moderate amounts of cytoplasm and containing pink to red colloid-like secretions. The uniform bland cells result in mostly low nuclear grading. This unique entity is TTF-1 and thyroglobulin negative, and immunostains should be performed to rule out a metastatic thyroid carcinoma before rendering a diagnosis. Immunohistochemically, they are mostly, but not always, CK7, CD10, Pax2, RCCm, AMACR, vimentin, ksp-cadherin, WT-1, and c-KIT/CD117 negative. Although papillary architecture is not commonly seen in this entity, it has been described in 1 report. In comparison, PRCC is CK7, AMACR, and RCCm positive, and c-KIT/CD117, ksp-cadherin, parvalbumin, and WT-1 negative. Oncocytomas and RCC, unclassified (“low-grade oncocytic type”, which is CK7 positive), may also show focal thyroid-like follicular features.
Epithelioid Angiomyolipoma Versus High-Grade Renal Cell Carcinoma Unclassified, Renal Cell Carcinoma with Sarcomatoid Differentiation and Translocation-Associated Carcinoma
Epithelioid angiomyolipoma, commonly seen in tuberous sclerosis patients, have sheet-like or alveolar architecture composed of 2 types of cells—clear cells with fine granular cytoplasm and small monomorphic nuclei, and eosinophilic cells with abundant cytoplasm, epithelioid morphology and large nuclei with prominent nucleoli. Focal fat and dysmorphic cells are present. The latter high-grade–type cells, in addition to the presence of mitoses, necrosis, extrarenal extension, renal vein involvement, and, rarely, distant metastases, can lead to misdiagnoses as high-grade RCC, unclassified. Useful immunostains include Melan-A and HMB-45 positivity in epithelioid angiomyolipoma, pancytokeratin, EMA, and Pax 8 positivity in RCC unclassified and RCC with sarcomatoid differentiation. MiTF/TFE translocation-associated carcinoma shares a similar immunoprofile of positive Melan-A and HMB-45, and negative epithelial markers but is variably positive for Pax 8. As mentioned, TFE3 and TFEB immunostains and molecular studies can help to provide a definitive diagnosis.
Papillary Neoplasms with Eosinophilic Features Versus Collecting Duct Carcinoma and Urothelial Carcinoma
Diagnosing PRCC is usually not a challenge on biopsies. However, type 2 PRCC lacks the foamy macrophages and intracellular hemosiderin accumulation. High-grade cytologic atypia, eosinophilic cytoplasm, and nuclear pseudostratification are common features. Collecting duct RCC (CDRCC) and urothelial carcinoma involving the renal pelvis are differentials to consider as variable papillary growth is seen. Other morphologic features of CDRCC, such as a neutrophil-rich infiltrate and dysplastic changes in adjacent renal collecting ducts, if present, are helpful. Of note, a pseudopapillary pattern can be seen in CCRCC from cell drop-off in areas away from feeding vessels.
PRCC is positive for CK7 (type 1 more often than type 2), CD10 (luminal membranous), AMACR (cytoplasmic granular), RCC and Pax 2; either negative or focal positive for CAIX in the papillary tips or perinecrotic areas. In contrast with PRCC, CDRCC are RCC antigen and AMACR negative. Urothelial carcinoma is CK20 and ULEX-1 positive, which is not seen in PRCC or CDRCC (see Table 1 ).
Hereditary leiomyomatosis-related RCC (HLRCC) shows variable but prominent papillary architecture, admixed with cystic/tubular, solid, or cribriform patterns. Very prominent macronucleoli and perinuclear halos are key distinct morphologic features. The presence of desmoplasia and multinodularity resembles CDRCC. Cytogenetics testing for fumarate hydratase germline mutation is confirmatory. An oncocytic variant with low-grade nonoverlapping nuclei with molecular and biologic similarity to type 1 PRCC has been described.
Succinate Dehydrogenase Mutant Renal Cell Carcinoma
RCC with SDHB deficiency is currently considered a provisional entity in the 2013 International Society of Urologic Pathology Vancouver Classification of renal tumors. However, it begs mention because it presents as part of a syndrome that might not yet be known in the patient at the time of biopsy. Those tumors with low nuclear grade features are not associated with a true oncocytic cytoplasm, but rather have flocculent cytoplasm. However, in the largest reported series, a few of the tumors described had International Society of Urologic Pathology nucleolar (nuclear) grade 3 cells, had high-grade areas with dense eosinophilic cytoplasm, and nested, solid sheets or focal abortive papillary architecture. These tumors, aside from SDHB negativity, show SDHA, Pax 8, and limited focal EMA positivity. Rare focal CK7 staining is seen. Most cases are completely negative for cytokeratins (AE1/AE3, CK8/18, CK7, CK20) and c-KIT/CD117, the latter which mainly highlights the characteristic intratumoral mast cells.
A recent case report described the first SDHA mutant RCC, which was associated with aggressive behavior. The tumor architecture was a combination of high-grade papillary and collecting duct carcinoma with pale eosinophilic cytoplasmic inclusions. Although SDHA-deficient tumors are not well-described, staining for both SDHB and SDHA should be performed when the differential is being considered.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





