Fig. 3.1
Normal esophagus: whitish pink mucosa and branching vessels aligned as a network in the horizontal plane
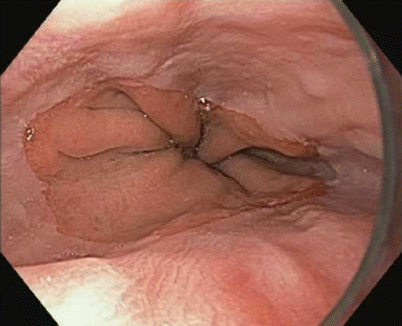
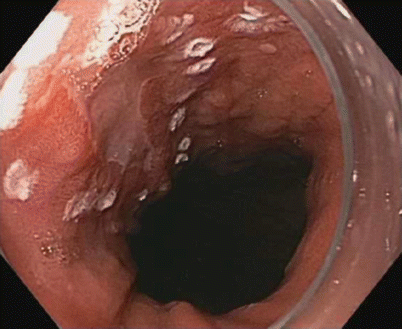
Fig. 3.2
Reflux esophagitis: one or more mucosal breaks less than 5 mm in maximal length (LA classification A)
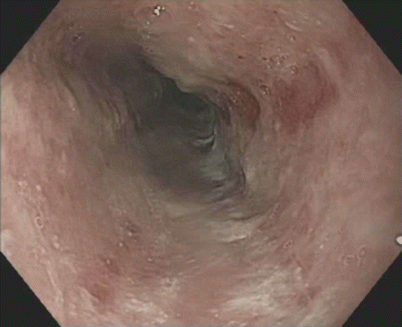
Fig. 3.3
Reflux esophagitis: mucosal breaks involving more than 75 % of the esophageal circumference (LA classification D)
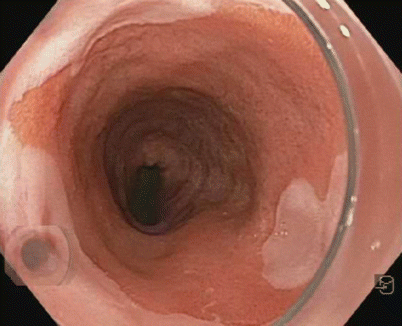
Fig. 3.4
Barrett’s esophagus: salmon-colored mucosa projecting from the gastroesophageal junction
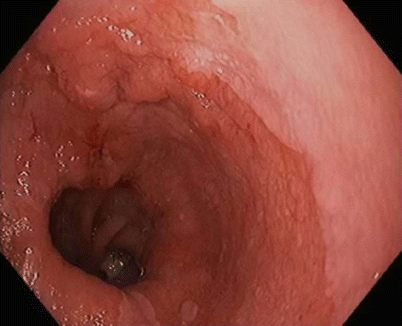
Fig. 3.5
Focal nodular lesion within the Barrett’s esophagus
3.2 Preoperative Workup
The first step is careful examination with high-resolution endoscopy to visualize the area of the target lesion and identify the margins of the neoplasia (Fig. 3.6). The updated Paris classification categorizes superficial lesions in the esophagus into a number of categories [9]:
Protruding pedunculated (type 0-Ip)
Protruding sessile (0-Is)
Slightly elevated (0-IIa)
Completely flat (0-IIb)
Slightly depressed (0-IIc)
Excavated (0-III)
Mixed pattern
We often need to enhance the mucosal lesion by using chromoendoscopy. Indigo carmine, a topical contrast agent, is the only dye that is not absorbed by the mucosa; absorptive dyes are less commonly used because some are considered carcinogenic, as they bind to the DNA within cells. Optical chromoendoscopy with narrow-band imaging (NBI) is now widely used (Fig. 3.7). This technique uses a narrow-band illumination of the light source to highlight mucosal structures and vascular patterns [10].
Endoscopic ultrasound (EUS) is usually performed to identify the depth of the lesion. Currently, EUS is considered the most accurate modality for preoperative locoregional staging, especially for the T stage of esophageal cancer (Figs. 3.8 and 3.9). It has been suggested that a combination of EUS and CT–positron emission tomography (PET) improves preoperative staging of esophageal cancer [11]. Even with high-frequency ultrasound probes, however, it can be difficult to distinguish between lesions confined to the mucosa and cancer invading the submucosa [12]. Another weak point is reproducibility and validity. Although EUS is often performed as part of clinical practice for all neoplastic lesions, it can be argued that EUS is not necessary in patients with very superficial cancers.
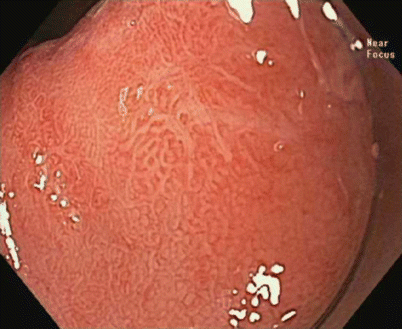
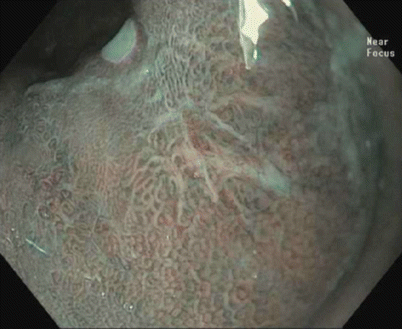
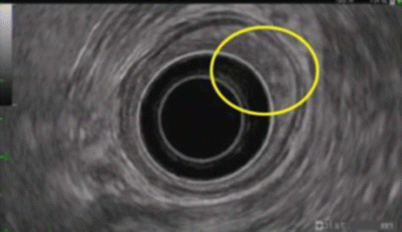
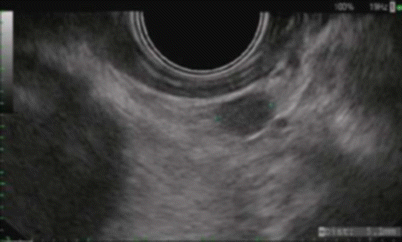

Fig. 3.6
High-resolution endoscopy with near-focus magnification, showing a type IIc mucosal lesion (updated Paris classification) within the Barrett’s esophagus

Fig. 3.7
NBI chromoendoscopy with magnification, showing type IIc mucosal lesion (updated Paris classification) within the Barrett’s esophagus

Fig. 3.8
Endoscopic ultrasound (EUS): hypoechoic mass extending into the submucosa, causing irregularity

Fig. 3.9
EUS: paraesophageal lymph node (oval, hypoechoic, well-defined margin)
3.3 Endoscopic Mucosal Resection
Normal saline solution with the addition of epinephrine (1:100,000–1:200,000) is widely used for injection-assisted EMR. The volume of injected solution ranges from 5 to 50 mL, depending on the size of the lesion. Repeated injections may be required if the cushion dissipates before complete resection of the lesion. Observation of the lesion during submucosal injection is important because it helps us to decide whether to proceed with EMR. Failure of the lesion to be lifted is often the result of submucosal neoplastic invasion or submucosal fibrosis from prior mucosal resection, and those lesions should be considered for alternative treatments. The most common way to perform EMR is either using cap-assisted EMR (C-EMR) or band-ligation EMR. C-EMR requires a transparent plastic cap on the tip of an endoscope. After submucosal injection, a crescent-shaped snare must be opened and positioned on the internal circumferential ridge at the tip of the cap. The preloop can be performed by lightly pressing against and suctioning normal mucosa to seal the cap outlet. The endoscope is then positioned immediately over the target lesion and the lesion is sucked into the cap, after which the snare is closed and cautery current is applied to cut the tissue (Figs. 3.10 and 3.11). After C-EMR, the resected specimen can be collected into the cap. In band-ligation EMR, suction is applied to retract the lesion into the banding device, and the lesion is ligated by placing a band at the bottom, with or without submucosal injection. An ha snare is then placed under the band and is used to cut the lesion.
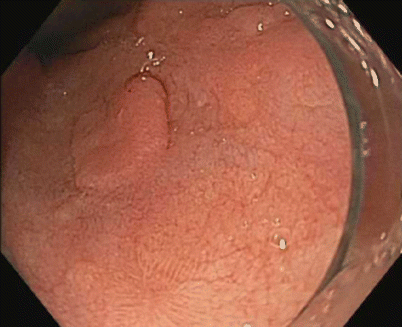
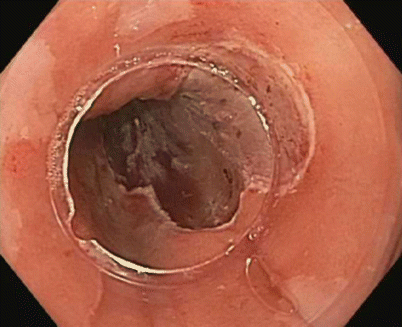

Fig. 3.10
Nodular lesion within the Barrett’s esophagus, before endoscopic mucosal resection (EMR)

Fig. 3.11
Complete mucosal resection and resulting scar after EMR of the nodular lesion
3.4 Endoscopic Submucosal Dissection
A forward-viewing, single-channel endoscope with a distally attached transparent cap on the endoscope tip and carbon dioxide for insufflation is utilized. The area of interest is first carefully marked using cautery, argon plasma coagulation (forced APC 70 W, flow 2.0 L/min), to mark 0.5 mm from the lesion’s lateral borders, as once the resection begins it is difficult to determine the boundaries of the lesion that needs to be removed (Fig. 3.12). Then, an indigo carmine solution mixed with 0.3 % hypomellose and saline is injected into the submucosal layer to separate it from the muscularis mucosa (Fig. 3.13). The lifting solution is injected via an injection needle. Subsequently, the mucosa is cut in a circumferential fashion using a dissecting knife to grab and then cut through the submucosal fibrous tissue. Injections are repeated as needed. Cautery is most commonly applied with a blended current with primary coagulation. Control of bleeding is obtained with coagulation via an electrocautery knife and hot biopsy forceps as well as hemostatic clips, or with a combination (Fig. 3.14). No type of knife has been clearly shown to be superior to the others.