Primary tumor (T)
Tx: Primary tumor cannot be assessed
T0: No evidence of primary tumor
Ta: Noninvasive papillary carcinoma
Tis: Carcinoma in situ (i.e., flat tumor)
T1: Tumor invades subepithelial connective tissue (lamina propria)
T2: Tumor invades muscle
pT2a: Tumor invades superficial muscle (inner half)
pT2b: Tumor invades deep muscle (outer half)
T3: Tumor invades perivesical tissue
pT3a: Microscopically
pT3b: Macroscopically (extravesical mass)
T4: Tumor invades any of the following: prostate, uterus, vagina, pelvic wall, or abdominal wall
T4a: Tumor invades the prostate, uterus, vagina
T4b: Tumor invades the pelvic wall, abdominal wall
Regional lymph nodes (N)
Nx: Regional lymph nodes cannot be assessed
N0: No regional lymph node metastasis
N1: Metastasis in a single lymph node, =2 cm in greatest dimension
N2: Metastasis in a single lymph node, >2 cm but =5 cm in greatest dimension; or multiple lymph nodes, =5 cm in greatest dimension
N3: Metastasis in a lymph node, >5 cm in greatest dimension
Distant metastasis (M)
Mx: Distant metastasis cannot be assessed
M0: No distant metastasis
M1: Distant metastasis
Urothelial papilloma |
Grade 1 (G1)—well differentiated |
Increase in number of urothelial cell layers, some loss of normal cellular orientation. No invasion |
Grade 2 (G2)–moderately differentiated |
Increased mitotic activity and loss of cellular polarity |
Nuclei more abnormal and show variable staining |
Grade 3 (G3)—poorly differentiated |
Cells very poorly differentiated with loss of cellular cohesion. Invasion often seen |
Normal |
Normal—may include cases formerly “mild dysplasia” |
Hyperplasia |
Flat |
Papillary |
Flat lesions with atypia |
Reactive (inflammatory) |
Atypia of unknown significance |
Dysplasia (low grade intraurothelial neoplasia) |
Carcinoma in situ (high grade intraurothelial neoplasia) |
Papillary neoplasms |
Papilloma |
Inverted papilloma |
Papillary neoplasm of low malignant potential (PUNLMP) |
Papillary carcinoma, low grade |
Papillary carcinoma, high grade |
Invasive neoplasms |
Lamina propria invasion |
Muscularis propria (detrusor) invasion |
Accurate histological diagnosis and staging ensures appropriate treatment, and in NMIBC, complete tumor clearance reduces ‘recurrence’ and improves the effectiveness of adjuvant intravesical therapies [17]. A ‘radical’ TURBT is not recommended as monotherapy for MIBC, but ‘maximal’ TURBT forms part of contemporary multimodal bladder preservation therapy for MIBC. A complete TURBT has been demonstrated to increase the rate of complete response to chemoradiotherapy (from 63 to 74 %) and reduce the need for salvage radical cystectomy (from 50 to 29 %) [18, 19].
The overall incidence of TURBT complications was 5.1 % of 2,821 patients in one study [20], but rises with increasing tumor size and multifocality. Bladder perforation is one of the most devastating complications. Open surgery for bladder perforation was required in 15 of 4,144 (0.36 %) patients in one retrospective cohort series, two of whom died of the iatrogenic injury [21]. Although carrying out traditional monopolar (MP) TURBT under full muscle paralysis, not overfilling the bladder, and use of short bursts of diathermy, can reduce the incidence of obturator nerve stimulation which can lead to bladder perforation, this can be avoided by the use of alternative energy sources.
14.3 Evolving TURBT
Bipolar TURBT (BP-TURBT)
Monopolar (MP-) TURBT has been the gold standard operation for bladder cancer. BP-TURBT is inherently safer, by reducing the chance of obturator nerve stimulation and by better hemostasis. Using a similar resectoscope and loop, plasmakinetic BP-TURBT uses transferable skills giving a short learning curve.
MP-TURBT and BP-TURBT have been compared in randomized [22–24] and non-randomized studies [25]. Those receiving BP-TURBT had no/lower risk of bladder perforation and a day shorter catheterization and hospitalization times. One group found that bladder perforation with BP-TURBT, was more likely with higher power, and abolished it by reducing the energy setting from 160 W cut /80 W coagulation to 50/40 W [26]. BP-TURBT may have advantages beyond less morbidity. In one randomized study less residual tumor was found in the bipolar group (9.3 % vs. 20.8 %) [24]. One could speculate that improved hemostasis and less char allows better tumor visibility, allowing more thorough resection. No difference in the quality of bladder tumor chips submitted for histological evaluation was found when MP-TURBT and BP-TURBT specimens were compared blindly [27].
Bipolar plasmavaporization of bladder tumors with the button ‘mushroom’ electrode instead of a resecting loop, is an ablative technique which may be useful for managing the bulk of large volume tumors or small recurrences [24, 28]. Biopsies of the tumor and tumor base are required as no tissue specimen is obtained using this technique.
The En-bloc Resection
Another possible recurrence mechanism is that traditional piecemeal TURBT may scatter tumor cells into the bladder lumen from where they could implant into the freshly cut and other urothelial surfaces [29]. Adjuvant single shot chemotherapy, as recommended for all patients by the EAU guidelines [16], was conceptualized to prevent cell implantation, thus overcoming this TURBT shortcoming [30]. En-bloc bladder tumor resection with a margin of normal tissue refines the surgical technique itself, for a more oncologically sound operation and may reduce NMIBC recurrence while providing a better a surgical specimen [31].
The EAU guidelines recommend that tumors <1 cm should be resected en-bloc [16]. A literature review reveals diverse techniques of en-bloc resection, but also that larger tumors can safely be managed thus.
En-bloc resection was described in 1997 using a bespoke arched resection electrode [32]. Other groups have used a knife electrode, a J-shaped needle, or more recently, the Holmium laser [33–36]. One group carried out en-bloc resection of tumors measuring 0.5–4.5 cm using the Collins knife in 41/46 (89 %) consecutive patients. The five tumors that could not be resected en-bloc were at the bladder neck [37]. Another group reported en-bloc polypectomy using a 7Fr monopolar lasso-like snare electrode passed through a cystoscope for tumors <5 cm. A margin of en-bloc normal bladder wall cannot be excised with the tumor by this method, and some tumors required bisection in order to be removed, but it was suggested that the majority of specimens could be withdrawn with the cystoscope or within a mesh net, reducing tumor cell scatter [38]. Fritsche combined a water jet dissector and needle knife (HybridKnife). The water jet at 30 atm elevated the tumor on the superficial layer of the muscularis propria on a bleb of saline thus facilitating en-bloc resection. The users found it easy to learn, with no additional morbidity [39].
Perhaps the most interesting technique is bladder tumor excision using an end firing laser fiber. It is a precise surgical tool, which negates obturator nerve stimulation risk. Both Holmium and Thulium lasers have been used for bladder cancer. Several small studies found Holmium laser bladder tumor resection (HoLBRT) to be technically safe and histologically uncompromised [33, 40–42]. A large non-randomized comparison of 101 HoLBRT and 111 MP-TURBT in primary tumors found operative time to be longer, but fewer complications, and shorter catheterization and hospitalization by 1 day, in the HoLBRT group [43]. En-bloc resection was possible in 84.9 % of 152 tumors, except for anterior wall tumors. Although the study was not designed to detect a difference in recurrence rates, after mean follow up of 34 months, during which all patients received adjuvant intravesical chemotherapy, no difference in recurrence was seen.
In a small six patient study, all histological specimens obtained after Thulium laser bladder tumor resection included deep muscle layer sampling, and there was no residual tumor at re-resection 6 weeks later [44].
Lasers are probably the best surgical tools for en-bloc bladder tumor resection. The current literature describes HoLBRT as a safe technique but efficacy evaluation in large prospective randomized trials is lacking.
14.4 Adjuncts to TURBT: Improving Tumor Detection
Significant evidence exists to prove that after an apparently visually complete resection, residual tumor frequently remains. A second, or re-resection, for patients with high-risk bladder cancer manages some of this residual disease, and in patients with low-risk disease, residual cancer may be detected as ‘recurrence’ at the first 3 month check cystoscopy. The more contemporary approach however, is to try to improve the first operation.
Photodynamic Diagnosis (PDD)
In the 1970s Kelly and Snell used PDD successfully to detect bladder tumors in cystectomy specimens using intravenous hematoporphyrin derivative and ultraviolet (UV) wavelength 390–420 nm light [45]. This technique utilized the heme biosynthetic pathway (Fig. 14.1). Kriegmair and colleagues, carried out a series of groundbreaking clinical studies using an intravesical photosensitizer 5-aminolevulinic acid (5ALA) in the 1990s [46–49]. This was the beginning of the modern era of clinically feasible PDD for bladder cancer.
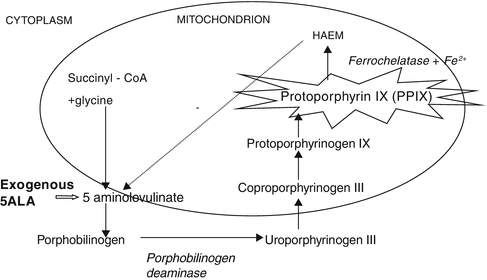

Fig. 14.1
Heme biosynthetic pathway. When the negative feedback mechanism of heme on 5ALA is overcome by exogenous 5ALA the fluorescent molecule protoporphyrin IX (PPIX) accumulates selectively in tumor cells
The third generation, regulatory approved, photosensitizer is hexylaminolevulinate (HAL). HAL causes tumors to fluorescence red contrasting against blue normal urothelium (Fig. 14.2). In addition to a safe, effective photosensitizer administered intravesically, PDD also requires a UV light source, narrow fluid light cables, a cystoscope modified with filters, and a CCD camera which allows the switch between white and blue light.
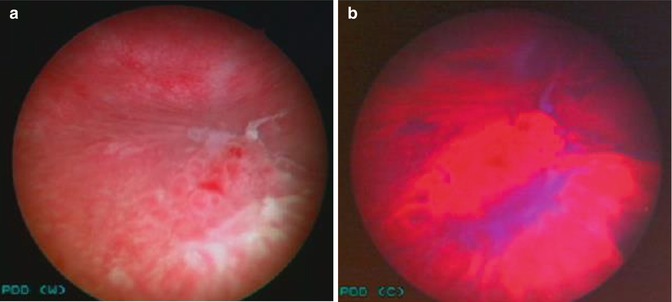

Fig. 14.2
Photodynamic diagnosis with hexylaminolevulinate. (a) White light cystoscopy, (b) PDD assisted cystoscopy gives a clearer indication of the extent of the tumor: histology low grade G2pTa + carcinoma in situ
A systematic review of the literature including 27 studies and 2,949 patients confirmed that PDD using either 5ALA or HAL had higher sensitivity for tumor than white light cystoscopy (WLC) (92 % vs. 71 %), but lower specificity (57 % vs. 72 %) at both patient and biopsy levels [14]. This difference was most pronounced for high-risk tumors where at the biopsy level, median sensitivity was 99 % vs. 67 %. Most importantly, improved tumor detection translated into reduced residual tumor and recurrence at 2 years. PDD assisted MP-TURBT resulted in statistically significantly fewer residual tumors [RR 0.37 (95 % CI, 0.20–0.69)]; and a small but statistically significant improvement in recurrence free survival (RFS) [RR 1.37 (95 % CI, 1.18–1.59)].
An unpublished meta-analysis of eight HAL-PDD studies including 2,231 patients found similar results. HAL-PDD detected 13.2 % more pTa tumors, 39.8 % more CIS lesions, and 24.6 % more CIS patients. Patients with new bladder cancer and recurrence benefitted. Recurrence at 12 months was lower in the PDD group at 34.5 % compared with 45.4 % in the WLC group [50]. Denzinger reported 80 % RFS at 8 years after PDD for patients with T1 high grade NMIBC compared with 52 % after WLC [51] suggesting a durable effect on recurrence. For progression, although PDD assisted TURBT slightly favored WLC, patient numbers were small, confidence intervals were wide, and the differences were not statistically significant [14, 52, 53].
Despite these convincing meta-analyses, recent studies have challenged the efficacy of PDD. Schumacher found high recurrence rates with no statistically significant difference at 1 year after WLC or 5ALA-PDD (53.1 % vs 50.4 %) [53].
Additionally, two trials using HAL and a routine single dose of intravesical chemotherapy for all patients found conflicting outcomes. A UK randomized trial of patients newly presenting with bladder cancer failed to show a statistically significant difference in 1 year recurrence after WLC or HAL-PDD (20 % vs. 15 %) [54]. However, in the trial by Karaolides, large differences in recurrence were seen at 18 months after WLC and HAL-PDD (49.4 % vs. 17.5 %) [55].
The improved detection of CIS is one of the undisputed attributes of PDD. Sensitivity ranges 92–97 % for HAL-PDD compared with 58–68 % for WLC [52, 56–58]. PDD should be considered for all patients with high grade cancer cells in the urine and an apparent absence of visible tumor in the bladder at WLC.
PDD undoubtedly improves tumor detection at the level of the urothelium: at the resection margin, satellite lesions, multifocal tumor, and CIS. What is still not categorically clear is whether PDD-assisted resection results in reduced long term recurrence. The results of good quality randomized trials, with and without adjunctive routine instillation chemotherapy and early re-resection, have demonstrated conflicting evidence. Although easy to learn, PDD requires a significant economical investment, while its clinical effectiveness is still to be completely realized.
Narrow Band Imaging
Digital imaging allows the incorporation of other technologies such as Narrow Band Imaging (NBI). White light is filtered to produce 415 nm blue and 540 nm green wavelengths which are absorbed by hemoglobin. On the NBI image blood vessels, which are more numerous in tumors, appear black, increasing the contrast between tumor and normal urothelium (Fig. 14.3). Bryan et al. first published results on the use of NBI in flexible cystoscopic assessment of recurrent NMIBC in 2008 and found it to be a technique which was easily adopted by new users [59, 60]. NBI, like PDD, can assist endoscopic tumor detection by achieving adequate resection margins.
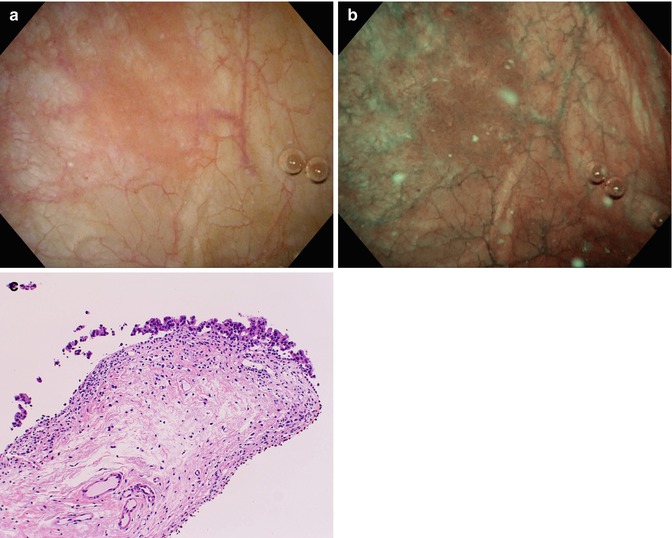

Fig. 14.3
Narrow Band Imaging (NBI) of area of bladder Carcinoma in situ (a) White light Cystoscopy, (b) NBI, (c) histology of targeted biopsy (Courtesy of Prof. Seiji Naito, Dept. Urology, Kyushu University, Fukuoka, Japan)
A systematic review and meta-analysis of NBI assisted WLC in 1,040 patients demonstrated that NBI detected an additional 17 % bladder cancer patients with an additional 24 % tumor detection [61, 62], and an additional 28 % CIS detection. Two prospective randomized studies have assessed the effect of NBI on bladder tumor recurrence [23, 63]. Geavlete found that residual tumor was significantly lower in the groups assessed with NBI (6.3 % vs. 17.5 %), which translated into significantly lower recurrence at 1 year (7.9 % vs. 17.8 %) [23]. Naselli found that after NBI, recurrence was significantly lower than in the WLC group at 3 months (3.9 % vs. 16.7 %) and at 1 year (32.9 % vs. 51.4 %) [63]. NBI was also found useful for the assessment patients with positive urine cytology for cancer, but no visible WLC disease (5/12 [42 %] had bladder cancer detected by NBI) [64].
These results are comparable to PDD, but NBI is potentially more cost-efficient as it requires only cystoscopes with NBI capability, in contrast to PDD, which involves preparation, time, and consumables.
14.5 Adjuncts to TURBT: Real Time Assessment of Bladder Lesions
Optical Coherence Tomography (OCT)
The use of OCT (Niris System: Imalux) was first reported in the human bladder in 1997 [65]. It uses near infrared light interferometry to visualize tissue microstructure in cross section. A 2.7 mm diameter OCT fiber passed through the cystoscope allows visualization of a small field of view with 2–3 mm penetration depth down to the muscularis propria. OCT offers the possibility of real time bladder lesion assessment detected by WLC, PDD, or NBI. In one small study, it was possible to differentiate between normal bladder, chronic inflammation, squamous metaplasia, severe dysplasia, and urothelial carcinoma [66]. It may also be valuable in the assessment of the extent of tumor depth, complimenting PDD and NBI, which assess the tumor lateral extent, for more complete resection.
In studies comparing OCT and histopathology, the sensitivity for cancer was 100 %; for detection of invasion of the lamina propria 90–100 %; and for detection of muscle invasive cancer 100 %, with a specificity of only 65–89 % [67–70].
Schmidbauer found that by combining PDD and OCT the specificity of PDD alone was increased from 78.6 to 97.9 % [71]. High magnification cystoscopy is a newer technique of NBI which can be used to look for neoangiogenesis and vascular patterns characteristic of a neoplasm, on suspect lesions [72]. OCT is not in common clinical use yet.
Raman Spectroscopy
This is a technique in which the Raman effect of light is used to assess the molecular composition of a bladder lesion. Like OCT it also has the potential for real-time assessment of bladder lesions. However, although it has shown some success in the assessment of cancer cell grade in urine [73], and the differentiation of cancerous from benign bladder biopsies with 84 % accuracy [74], only ex-vivo studies exist. In vivo endoscopic use has been unsuccessful due to poor specificity, but newly developed confocal Raman probes show some promise ex-vivo [75].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








