Treatment of metastatic renal cell carcinoma (mRCC) has evolved dramatically within the past 10 years with the advent of therapy targeting the angiogenesis and mammalian target of rapamycin (mTOR) pathways. These therapies rapidly supplanted immunotherapy as a first-line systemic treatment option. Response rates, however, continue to vary, largely due to mRCC’s clinical and molecular heterogeneity. This article reviews current understanding of mRCC biology and available treatments, discusses novel biomarkers that improve prognostication and may be able to predict response, and integrates available literature on surgical and systemic therapies into an individualized strategy.
One of 3 cases of renal cell carcinoma (RCC) is diagnosed with metastases at the time of initial presentation. In addition, 20% to 40% of patients treated for local disease experience metachronous metastases. Once RCC has metastasized, the chance of durable complete response is low. Because RCC is traditionally viewed as chemotherapy resistant and radiotherapy resistant, early treatments relied on cytokine therapy, which had low response rates and high levels of treatment-related toxicity. Over the past 6 years, the armamentarium for treating metastatic RCC (mRCC) has increased greatly with the emergence of targeted therapy directed against angiogenesis and mammalian target of rapamycin (mTOR) pathways.
There are several factors that make treatment of mRCC well suited for an individualized approach. The first is its biologic diversity. The variability in response rates exemplifies differences in both tumor biology and host response to the tumor and therapy. Second, improvements are being made in the ability to recognize individual differences in mRCC, both clinical and molecular. Lastly, therapies against mRCC are numerous, allowing for adjustment of treatment.
The personalized approach to cancer therapy is based on the specific biology of either the tumor or the host. An example of this type of care is the use of HER2/neu in breast cancer. Patients who have expression of this receptor are treated with a monoclonal antibody against HER2/neu, which has been shown to improve survival. This approach to treating cancer offers more efficacious therapy while limiting the treatment-related toxicity in nonresponders.
This article addresses the improved understanding of RCC biology and the molecular pathways involved in tumorigenesis, reviews available surgical and systemic therapies as well as risk stratification tools and biomarkers that assist in prognostication and prediction of response, and, lastly, reviews the available data that can guide systemic therapy and surgery to a more individualized strategy.
Relevant biologic pathways in RCC
Tumor hypoxia is a common feature in solid tumors, such as RCC, and is associated with poor patient outcomes. Hypoxia is important in tumor progression because it has the potential to limit cell proliferation and differentiation while promoting necrosis and apoptosis. Its presence can also lead to more aggressive tumors with abundant angiogenesis. RCC is a tumor that is known for its marked vascularity, and investigation into its biology has uncovered hypoxia-induced signaling as a main element in tumorigenesis and progression. Fig. 1 provides an overview of the biologic pathways in RCC.
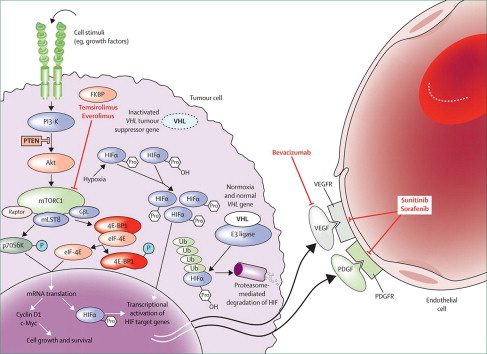
Important in understanding the angiogenesis pathway for RCC is the identification of the von Hippel-Lindau ( VHL ) gene as a critical modulator of hypoxia-responsive gene elements. VHL is a tumor suppressor gene that encodes for the VHL protein. This protein complexes with cullin 2, elongin B, and elongin C to form the E3 ubiquitin-ligase complex. In normoxic conditions, the ubiquitin-ligase complex targets hypoxia-inducible factors (HIF-1α and HIF-2α) for ubiquitin-mediated degradation by hydroxylation. In hypoxic conditions, HIF-1α and HIF-2α do not undergo hydroxylation and act as transcription factors for more than 200 genes. Proteins regulated by HIF include vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF). Mutation of the both VHL genes causes defective complex formation. With an ineffective ubiquitin-ligase complex, HIF levels accumulate and facilitate the transcription of genes involved in angiogenesis, cell survival, and cell proliferation.
HIF can receive input from another key cellular pathway: mTOR. This pathway is established as important in the regulation of multiple oncologic processes, such as cell survival and angiogenesis. mTOR is a serine/threonine kinase involved in cell response to energy depletion and hypoxia. mTOR up-regulation is implicated in both chemotherapy and radiotherapy resistance. Through immunohistochemical analysis, mTOR has been found up-regulated in RCC compared with normal renal tissue. After binding of VEGF, PDGF, or other growth factors to a receptor tyrosine kinase, phosphatidylinositol 3-kinase (PI3K) is activated. Protein kinase B (Akt) is recruited and able to activate mTOR. mTOR can also be activated Akt by phosphorylation. Akt can then inhibit cell apoptosis by inactivating proteins, such as procaspase-9 and AKD1. mTOR is also able to activate ribosomal protein S6 kinase, which has broad effects on cell physiology and survival. Increased S6 kinase expression is associated with more aggressive RCC.
Overview of treatments of mRCC
Surgery
Despite mRCC being a systemic disease, surgery continues to play a role in its optimal treatment. Traditionally, this role was reserved for palliation in patients with symptoms of pain or bleeding. Given the lack of effective systemic therapy, many patients underwent cytoreductive nephrectomy (CN) with the presumption that removing a large portion of the tumor burden would improve response to systemic therapy. Although rare, spontaneous regression of metastases was seen after CN, indicating a beneficial biologic response to the surgery. There are hypotheses for CN improving survival, the most prominent that the primary tumor suppresses the activation of T cells. By removing this suppression, the immune system has greater activity against sites of metastasis. Another possible mechanism is the beneficial removal of cells that produce tumor-related growth factors that result in abnormal signaling pathways.
Early experience with CN showed that morbidity from surgery precluded many patients from receiving immunotherapy. One study found that in patients with an Eastern Cooperative Oncology Group (ECOG) performance status ranging from 0 to 2, only 23% were able to undergo immunotherapy after CN. This pattern of treatment called into question the benefit of CN. Since then, however, 2 randomized trials have examined the survival after CN plus interferon-α versus interferon-α alone. The Southwest Oncology Group (SWOG) trial, which included 120 patients from 1991 to 1998, found that CN was associated with a statistically significant 3-month survival advantage (11.1 vs 8.1 months). The European Organisation for Research and Treatment of Cancer (EORTC) study, which randomized 85 patients from 1995 and 1998, reported a median survival benefit of 10 months associated with CN (17 vs 7 months). A combined analysis of the 2 trials found CN was associated with a 6-month median survival benefit (13.6 vs 7.8 months).
There are no randomized data that demonstrate a survival benefit for CN with systemic targeted therapy. Thus, many have extrapolated the benefit of CN from the experience with immunotherapy to targeted drugs. Another argument for CN’s role in the era of targeted therapy is that the study population for targeted therapy included mainly those who have had a prior nephrectomy. Because the vast majority of patients enrolled in these studies had undergone nephrectomy, it is difficult to translate the positive findings of the phase 3 trials to patients who do not undergo CN.
Metastatectomy for RCC was described in 1939 in a patient who lived 23 years after surgery, ultimately dying of coronary artery disease. In the absence of effective systemic therapy, metastatectomy was thought a reasonable approach to controlling systemic disease in select patients. Multiple studies have demonstrated favorable survival in select patients after judicious metastasectomy, with 5-year survival rates ranging from 35% to 60%. There are important caveats, however, to metastasectomy. First, it is unclear to what extent indolent cancer biology contributed to improved survival. Also, the role of metastatectomy in the targeted therapy era has yet to be clearly defined. Lastly, there are currently no prospective or randomized data that can accurately determine the survival impact of metastasectomy. Despite these limitations, metastatectomy remains a viable option in highly selected patients with mRCC.
Systemic Therapy
Immunotherapy was a standard systemic treatment of mRCC in the 1980s. Cytokine therapy remains the only treatment that offers a chance of achieving a durable complete response. The cytokines interferon-α and interleukin (IL)-2 are generally associated with low rates of response and high levels of toxicity. Interferon-α has a complete response rate of 2.5% and a partial response rate of 26%. IL-2 has a complete response rate of approximately 5% to 7% and a 15% to 20% overall response rate. In the past, high-dose IL-2 has been associated with treatment mortality as high as 4%. Better patient selection and supportive care have helped mitigate many of these toxicities.
Unlike immunotherapy, targeted therapy theoretically offers more specific sites of action and less toxicity (see Fig. 1 ). Tyrosine kinase inhibitors that target angiogenesis pathways include sunitinib, sorafenib, pazopanib, tivozanib, and axitinib, which are shown to improve either overall survival (OS) or progression-free survival (PFS) in various mRCC patient contexts.
In a randomized phase 3 study, sunitinib improved PFS compared with interferon-α in treatment-naïve patients (11 vs 5 months). Sorafenib as first-line therapy was found to have similar PFS compared with interferon-α in a phase 2 study. Sorafenib, however, had superior tumor control compared with interferon-α as well as a benefit in PFS in those who underwent dose escalation or crossover from interferon-α. Sorafenib as a second-line therapy has been shown to significantly improve PFS compared with placebo (5.5 vs 2.8 months). Pazopanib has been shown to improve PFS in treatment-naïve and immunotherapy-treated patients compared with placebo in a phase 3 trial (9.2 vs 4.2 months). Axitinib, a second-generation tyrosine kinase inhibitor with more potent VEGF inhibition, has been evaluated as a second-line treatment of mRCC with a recent randomized study reporting longer PFS compared with sorafenib (6.7 vs 4.7 months). Most recently, tivozanib was reported to improve PFS compared with sorafenib in the frontline setting.
There are several tyrosine kinases that remain in development. Dovitinib inhibits multiple receptors, including fibroblast growth factor receptor, which is implicated as an escape mechanism after VEGF-targeted therapy. Erlotinib is an inhibitor of the epidermal growth factor receptor, which has had a response rate of 11% in metastatic papillary RCC in a phase 2 trial. A follow-up trial of erlotinib plus the c-MET inhibitor ARQ 197 is in development by SWOG for advanced papillary RCC.
Another type of therapy targeting the VEGF receptor is bevacizumab, a monoclonal antibody against VEGF. One randomized, double-blind, phase 3 trial compared bevacizumab plus interferon-α to interferon-α plus placebo in treatment-naïve patients. This study reported that bevacizumab plus interferon-α significantly improved PFS compared with the control group (10.2 vs 5.4 months). Later analysis showed a numerically higher median OS with bevacizumab (23.3 vs 21.3 months), but this did not reach significance ( P = .129). A similar randomized phase 3 trial compared bevacizumab plus interferon-α to interferon-α monotherapy, again failing to find a significant improvement in OS with bevacizumab (18.3 vs 17.4 months, P = .97) but showing a higher response rate and improved PFS for the bevacizumab arm.
Temsirolimus and everolimus act by inhibiting mTOR and were Food and Drug Administration approved for the treatment of mRCC after the results of large phase 3 registration studies. Temsirolimus was evaluated in a multicenter, phase 3, randomized trial of temsirolimus versus interferon-α versus temsirolimus plus interferon-α in treatment-naïve, poor-risk patients that included 20% with non–clear cell histology. The trial reported that temsirolimus significantly improved PFS (5.5 vs 3.1 months) and OS (10.9 vs 7.3 months) compared with interferon-α. Everolimus was tested in a randomized, double-blind, placebo-controlled crossover trial as second-line therapy in patients who had progressed on tyrosine kinase inhibitors of VEGF receptor, such as sunitinib and/or sorafenib. Everolimus was shown to significantly prolong PFS (4.0 vs 1.9 months).
Overview of treatments of mRCC
Surgery
Despite mRCC being a systemic disease, surgery continues to play a role in its optimal treatment. Traditionally, this role was reserved for palliation in patients with symptoms of pain or bleeding. Given the lack of effective systemic therapy, many patients underwent cytoreductive nephrectomy (CN) with the presumption that removing a large portion of the tumor burden would improve response to systemic therapy. Although rare, spontaneous regression of metastases was seen after CN, indicating a beneficial biologic response to the surgery. There are hypotheses for CN improving survival, the most prominent that the primary tumor suppresses the activation of T cells. By removing this suppression, the immune system has greater activity against sites of metastasis. Another possible mechanism is the beneficial removal of cells that produce tumor-related growth factors that result in abnormal signaling pathways.
Early experience with CN showed that morbidity from surgery precluded many patients from receiving immunotherapy. One study found that in patients with an Eastern Cooperative Oncology Group (ECOG) performance status ranging from 0 to 2, only 23% were able to undergo immunotherapy after CN. This pattern of treatment called into question the benefit of CN. Since then, however, 2 randomized trials have examined the survival after CN plus interferon-α versus interferon-α alone. The Southwest Oncology Group (SWOG) trial, which included 120 patients from 1991 to 1998, found that CN was associated with a statistically significant 3-month survival advantage (11.1 vs 8.1 months). The European Organisation for Research and Treatment of Cancer (EORTC) study, which randomized 85 patients from 1995 and 1998, reported a median survival benefit of 10 months associated with CN (17 vs 7 months). A combined analysis of the 2 trials found CN was associated with a 6-month median survival benefit (13.6 vs 7.8 months).
There are no randomized data that demonstrate a survival benefit for CN with systemic targeted therapy. Thus, many have extrapolated the benefit of CN from the experience with immunotherapy to targeted drugs. Another argument for CN’s role in the era of targeted therapy is that the study population for targeted therapy included mainly those who have had a prior nephrectomy. Because the vast majority of patients enrolled in these studies had undergone nephrectomy, it is difficult to translate the positive findings of the phase 3 trials to patients who do not undergo CN.
Metastatectomy for RCC was described in 1939 in a patient who lived 23 years after surgery, ultimately dying of coronary artery disease. In the absence of effective systemic therapy, metastatectomy was thought a reasonable approach to controlling systemic disease in select patients. Multiple studies have demonstrated favorable survival in select patients after judicious metastasectomy, with 5-year survival rates ranging from 35% to 60%. There are important caveats, however, to metastasectomy. First, it is unclear to what extent indolent cancer biology contributed to improved survival. Also, the role of metastatectomy in the targeted therapy era has yet to be clearly defined. Lastly, there are currently no prospective or randomized data that can accurately determine the survival impact of metastasectomy. Despite these limitations, metastatectomy remains a viable option in highly selected patients with mRCC.
Systemic Therapy
Immunotherapy was a standard systemic treatment of mRCC in the 1980s. Cytokine therapy remains the only treatment that offers a chance of achieving a durable complete response. The cytokines interferon-α and interleukin (IL)-2 are generally associated with low rates of response and high levels of toxicity. Interferon-α has a complete response rate of 2.5% and a partial response rate of 26%. IL-2 has a complete response rate of approximately 5% to 7% and a 15% to 20% overall response rate. In the past, high-dose IL-2 has been associated with treatment mortality as high as 4%. Better patient selection and supportive care have helped mitigate many of these toxicities.
Unlike immunotherapy, targeted therapy theoretically offers more specific sites of action and less toxicity (see Fig. 1 ). Tyrosine kinase inhibitors that target angiogenesis pathways include sunitinib, sorafenib, pazopanib, tivozanib, and axitinib, which are shown to improve either overall survival (OS) or progression-free survival (PFS) in various mRCC patient contexts.
In a randomized phase 3 study, sunitinib improved PFS compared with interferon-α in treatment-naïve patients (11 vs 5 months). Sorafenib as first-line therapy was found to have similar PFS compared with interferon-α in a phase 2 study. Sorafenib, however, had superior tumor control compared with interferon-α as well as a benefit in PFS in those who underwent dose escalation or crossover from interferon-α. Sorafenib as a second-line therapy has been shown to significantly improve PFS compared with placebo (5.5 vs 2.8 months). Pazopanib has been shown to improve PFS in treatment-naïve and immunotherapy-treated patients compared with placebo in a phase 3 trial (9.2 vs 4.2 months). Axitinib, a second-generation tyrosine kinase inhibitor with more potent VEGF inhibition, has been evaluated as a second-line treatment of mRCC with a recent randomized study reporting longer PFS compared with sorafenib (6.7 vs 4.7 months). Most recently, tivozanib was reported to improve PFS compared with sorafenib in the frontline setting.
There are several tyrosine kinases that remain in development. Dovitinib inhibits multiple receptors, including fibroblast growth factor receptor, which is implicated as an escape mechanism after VEGF-targeted therapy. Erlotinib is an inhibitor of the epidermal growth factor receptor, which has had a response rate of 11% in metastatic papillary RCC in a phase 2 trial. A follow-up trial of erlotinib plus the c-MET inhibitor ARQ 197 is in development by SWOG for advanced papillary RCC.
Another type of therapy targeting the VEGF receptor is bevacizumab, a monoclonal antibody against VEGF. One randomized, double-blind, phase 3 trial compared bevacizumab plus interferon-α to interferon-α plus placebo in treatment-naïve patients. This study reported that bevacizumab plus interferon-α significantly improved PFS compared with the control group (10.2 vs 5.4 months). Later analysis showed a numerically higher median OS with bevacizumab (23.3 vs 21.3 months), but this did not reach significance ( P = .129). A similar randomized phase 3 trial compared bevacizumab plus interferon-α to interferon-α monotherapy, again failing to find a significant improvement in OS with bevacizumab (18.3 vs 17.4 months, P = .97) but showing a higher response rate and improved PFS for the bevacizumab arm.
Temsirolimus and everolimus act by inhibiting mTOR and were Food and Drug Administration approved for the treatment of mRCC after the results of large phase 3 registration studies. Temsirolimus was evaluated in a multicenter, phase 3, randomized trial of temsirolimus versus interferon-α versus temsirolimus plus interferon-α in treatment-naïve, poor-risk patients that included 20% with non–clear cell histology. The trial reported that temsirolimus significantly improved PFS (5.5 vs 3.1 months) and OS (10.9 vs 7.3 months) compared with interferon-α. Everolimus was tested in a randomized, double-blind, placebo-controlled crossover trial as second-line therapy in patients who had progressed on tyrosine kinase inhibitors of VEGF receptor, such as sunitinib and/or sorafenib. Everolimus was shown to significantly prolong PFS (4.0 vs 1.9 months).
Prognostication
Clinical Biomarkers
Risk stratification has emerged as an important clinical instrument useful in patient counseling, designing of clinical trials, and selecting appropriate therapies. Use of clinical markers for risk stratifications takes a broad view of a tumor’s biology and also takes into account individual response to the tumor. The most widely used risk criteria for mRCC were developed by Motzer and colleagues at the Memorial Sloan-Kettering Cancer Center (MSKCC). Five risk factors were identified as most prognostic in survival: low performance status, high lactate dehydrogenase, low serum hemoglobin, high corrected serum calcium, and time from initial RCC diagnosis to start of systemic therapy of less than 1 year. Patients with none of these risk factors are categorized as favorable risk, those with 1 or 2 risk factors have intermediate-risk disease, and those with 3 or more risk factors are categorized as poor risk. In a study examining these criteria in patients undergoing treatment with interferon-α, median survival was 30 months in the favorable-risk group, 14 months in the intermediate-risk group, and 5 months in the poor-risk group.
The MSKCC criteria have been externally validated and additional predictors of survival elucidated. A study of treatment-naïve patients with mRCC enrolled in clinical trials found prior radiotherapy and the presence of liver, lung, and retroperitoneal nodal metastases as independent predictors of survival. The other notable clinical prognostic criteria, the University of California Los Angeles Integrated Staging System, uses TNM staging, ECOG performance status, and Fuhrman grade. This system has also been validated and is associated with survival.
Prognostic factors for OS in patients treated with VEGF-targeted therapy were examined by Heng and colleagues in a study of 645 patients treated with VEGF therapy for the first time. There was agreement with 4 MSKCC criteria associated with worse survival: anemia, hypercalcemia, Karnofsky performance scale status (KPS) less than 80%, and time from diagnosis to treatment of less than 1 year. In addition, neutrophilia and thrombocytosis were identified as adverse prognostic factors. Of these 6 factors, patients were divided into risk categories: favorable (no prognostic factors), intermediate (1–2 prognostic factors), and poor (3–6 prognostic factors). Two-year OS rates for the groups were 75%, 53%, and 7%, respectively.
A similar study examined prognostic factors in patients with clear cell RCC (ccRCC) undergoing treatment with bevacizumab, sorafenib, sunitinib, or axitinib. Five factors were found associated with worse PFS on multivariate analysis: time from diagnosis to treatment of less than 2 years, baseline platelet count greater than 300 K/μL, baseline neutrophil count greater than 4.5 K/μL, corrected serum calcium less than 8.5 mg/dL or greater than 10 mg/dL, and initial ECOG performance status greater than 0. Using these clinical factors, 3 prognostic groups were formed with a median PFS that was 20 months in the favorable-risk group (0–1 factor), 13 months in the intermediate-risk group (2 factors), and 3.9 months in the poor-risk group (>2 factors).
Molecular Biomarkers
An emerging method of prognostication is with molecular biomarkers. These markers can be used to determine prognosis independently and can add to the accuracy of current prognostic models. Table 1 provides a summary of the many molecular markers in RCC and their association with various outcomes.
| Marker | Prognosis | PFS | OS | CSM | Treatment Efficacy | Added Value in Prognostic Models |
|---|---|---|---|---|---|---|
| Neutrophil | + | + (In IL-2 patients) | + | NS (low response rate in IL-2 patients) | + (Leibovich) | |
| C-reactive protein | + | + | ||||
| VHL | +/NS | +/NS | + | + (Predicts response to targeted therapy) | + | |
| HIF-α | + | + (Predicts response to sunitinib) | ||||
| Tissue based | ||||||
| VEGF | + | + | ||||
| CAIX | + | + | ||||
| mTOR | ||||||
| pS6 | + | + (Predicts response to temsirolimus) | ||||
| PTEN | + | + (Predicts response to mTOR inhibitors)/ns (low response in temsirolimus) | ||||
| pAkt | + (For localized and mRCC) | + | + (Predicts response to temsirolimus) | |||
| Other | ||||||
| Caveolin-1 | +/NS (coexpression with Akt/mTOR) | |||||
| p53 | NS | |||||
| Ki-67 | + | + | + (Coexpression with CAIX) | |||
| Survivin | + | + | ||||
| B7-H1 | + | + | ||||
| Vimentin | + | |||||
| Fascin | ||||||
| MMP | + | |||||
| IMP3 | + | + | + (SSIGN) | |||
| Blood based | ||||||
| VEGF | +/NS | +/NS (in mRCC)/+ (in sorafenib patients) | + (Predicts response rate in sunitinib)/ns (does not predict response rate in sorafenib) | |||
| CAIX | + | + | ||||
| NGAL | + | |||||
| SAA | + | |||||
| IGF-I | + | |||||
| NMP-22 | ||||||
A carbonic anhydrase enzyme, CAIX is one of the more notable markers given its specificity for RCC. It has not been identified in healthy renal tissue and is expressed in almost all cases of ccRCC. CAIX is involved in the reversible reaction that converts water and carbon dioxide to bicarbonate and a hydrogen cation. Increased levels of CAIX are necessary for pH balance in a tumor’s microenvironment of hypoxia and acidosis. Using immunohistochemistry and a threshold of 85% CAIX staining, Bui and colleagues found that lower CAIX levels were independently associated with poor survival in patients with advanced RCC. The reason for this association is not entirely clear, although the authors hypothesize that CAIX may be more important in the early stages of tumorigenesis to deal with hypoxia. Therefore, CAIX may not be as important in mRCC, which can account for the observed low levels of expression. Another study of patients with ccRCC found that low CAIX expression was associated with worsened survival, although it was not an independent predictor when adjusting for other factors, such as nuclear grade.
VEGF levels within the tumor can also be used as a prognostic marker. Opposite from CAIX, increased expression in tissue samples has been shown to predict worse survival. The combination of low expression of CAIX and high expression of VEGF was shown more accurate in prognostication than when evaluating these markers individually. Serum VEGF may also have a role in prognostication of mRCC, because these levels correlate well with tissue levels of VEGF. Jacobsen and colleagues examined serum VEGF before surgery and found increases associated with decreased survival. In mRCC, elevated serum VEGF has been correlated with decreased survival; however, VEGF levels were not independently prognostic on multivariate analysis.
HIF-1α has been studied as a marker for ccRCC because its expression is significantly elevated compared with benign renal tissue. Its increased nuclear expression has been shown a predictor of worse survival in metastatic disease (13.5 vs 24.4 months) on multivariate analysis. Other investigators examining cytoplasmic levels of HIF-1α, however, found high expression was associated with significantly longer survival. HIF-1α levels were not associated with survival in patients with non–clear cell histology or those categorized as poor risk. Further research into the role of HIF-1α, including emerging evidence that it has a role as a tumor suppressor, can help explain these reported differences.
A panel of molecular markers has been found an independent predictor of poor outcome in patients with ccRCC. The expression of B7-H1, survivin, and Ki-67 together formed a BioScore that is able to predicted RCC-specific death on multivariate analysis. Kim and colleagues examined tissue from 150 patients with metastatic ccRCC with tissue array examining expression of CAIX, phosphatase and tensin homolog (PTEN), vimentin, and protein 53 (p53). Using these markers, they were able to predict disease-specific survival on multivariate analysis. The corrected concordance index of this tissue array was superior to the University of California Los Angeles Integrated Staging System. When this group examined tissue from both localized and mRCC, they found the combination of clinical and molecular factors was a more prognostic than the either one independently.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






