Fig. 13.1
Pathophysiology of dumping syndrome
Late dumping, often occurring 2–3 h postprandially, involves mainly vasomotor complaints characterized by perspiration, palpitation, mental confusion, and sometimes syncope. Rapid delivery of a meal to the small intestine leads to an initial higher concentration of carbohydrates in the proximal small bowel, followed by rapid absorption of glucose into the blood. This is countered by the excessive release of insulin, the so-called “hyperinsulinemic response,” responsible for the subsequent reactive hypoglycemia [10]. The majority of patients exhibit early dumping, approximately 25 % of them exhibit late dumping, and only a minority of patients have symptoms of both [11].
Diagnosis
Dumping syndrome is diagnosed based on a group of symptoms in patients who have undergone gastric surgery, or by the dumping provocation test. In 1970, Sigstad [12] proposed a scoring system based on the occurrence of different symptoms of dumping syndrome, to calculate a diagnostic index (Table 13.1). A diagnostic index > 7 is suggestive of dumping syndrome. This system is simple to use, but its disadvantage is that it is difficult to distinguish other postprandial symptoms from dumping. The score index is helpful in clinical practice to assess response to therapy.
Table 13.1
Sigstad score. Weighting factors allocated to the symptoms and signs of dumping syndrome
Sigstad score | |
|---|---|
Preshock, shock | 5 |
Almost fainting, syncope, loss of consciousness | 4 |
Desire to lie or sit down | 3 |
Breathlessness, dyspnea | 3 |
Weakness, exhaustion | 3 |
Sleepiness, drowsiness, yawning, apathy, falling asleep | 3 |
Palpitation | 3 |
Restlessness | 2 |
Dizziness | 2 |
Headache | 1 |
Feeling of warmth, sweating, pallor, clammy skin | 1 |
Nausea | 1 |
Fullness in the abdomen, meteorism | 1 |
Borborygmus | 1 |
Eructation | − 1 |
Vomiting | − 4 |
A provocative test for assessing dumping syndrome can be used to confirm clinical suspicions. This test is a modification of the oral glucose tolerance test (OGTT) and involves the ingestion of 50 or 75 g glucose in solution after an overnight fast. Immediately before and up to 180 min after ingestion of this solution, the blood glucose concentration, hematocrit, pulse rate, and blood pressure are measured at 30 min intervals. The provocative test is considered positive if late (120–180 min) hypoglycemia occurs, or if an early (30 min) increase in hematocrit of more than 3 % occurs. The best predictor of dumping syndrome seems to be a rise in pulse rate of more than 10 bpm (beat per min) after 30 min [13]. Assessments of the speed of gastric emptying might show that this process occurs rapidly in patients with dumping syndrome—especially for liquid nutrients—but this test does not seem to have good diagnostic sensitivity or specificity, probably because rapid emptying occurs early after meal ingestion, a phase that is not analyzed closely or separately in most protocols that test gastric emptying [10, 13, 14].
Prevention
Prevention, rather than treatment, is recommended for dumping syndrome. The introduction of proton pump inhibitors and Helicobacter pylori eradication decrease the need for elective surgery in peptic ulcer disease. In addition, highly selective gastric vagotomy, which causes minimal disturbance of the gastric emptying mechanism, results in a lower incidence of dumping syndrome [15]. If more extensive surgery is necessary, a Roux-en-Y gastrojejunostomy (RYGJ) is preferable because of its decreased rate of dumping, when compared with pyloroplasty or loop gastrojejunostomy [16–18].
The choice of reconstructional method after distal gastrectomy is still controversial. The use of the Billroth I procedure after distal gastrectomy is preferred in Japan, whereas Billroth II is more common in Korea because it facilitates wider dissection and less anastomotic tension. There are some advantages in Billroth I compared to Billroth II, as follows: a more natural route for food passage, potentially less operative time due to one anastomosis, no risk of duodenal stump leakage, and less incidence of postoperative weight loss, anemia, and dumping syndrome. The disadvantage of Billroth I, however, is that the dissection area can be limited in order to facilitate a tension-free anastomosis. Therefore, the Billroth I procedure is commonly used for benign disease or distally located EGC in Korea. However, Kim et al. [19] compared results from 122 gastric carcinoma patients undergoing Billroth I and Billroth II gastrectomy. They evaluated postgastrectomy syndrome with a survey of abdominal symptoms, and dumping syndrome was measured using the Sigstad dumping score. According to their results, the occurrence of abdominal symptoms and dumping syndrome was lower in the Billroth I group than in the Billroth II group. Furthermore, pylorus-preserving gastrectomy (PPG) is a kind of reduced-gastric operation that preserves the distal portion (1.5 cm) of the gastric antrum and reduces postoperative complications such as dumping syndrome and reflux esophagitis [20]. However, a limitation of this operation is that complete lymph node (LN) dissection of the suprapyloric LN is undesirable for the preservation of the pyloric branch of the vagus nerve. Nowadays, some reports state that this procedure may be applicable in EGC confined to the mucosa and located at the gastric mid-body [21].
According to a recent Japanese large-scale investigation into dumping syndrome after gastrectomy for gastric cancer, [22] many more patients suffer from early dumping syndrome (67.6 %) than from late dumping syndrome (38.4 %) after gastrectomy. This study revealed that patients suffering from at least one symptom of early dumping syndrome were significantly more likely to also experience symptoms of late dumping syndrome. The study also demonstrated that two clinical factors, the surgical procedures used and the amount of weight loss, were significantly associated with the occurrence of both early and late dumping syndromes. Consistent with previous reports, [23, 24] patients who underwent PPG showed the lowest incidence of dumping syndrome. In addition, patients who underwent PG (proximal gastrectomy with jejunal interposition) showed the second highest incidence of early dumping syndrome. Patients who underwent RYGJ showed a lower incidence of dumping syndrome symptoms relative to Billroth I patients. Taken together, RYGJ or Billroth I is less associated with dumping syndrome after distal gastrectomy than Billroth II.
Management of Dumping Syndrome
The first step in treating dumping syndrome is the introduction of dietary modification. If this approach is insufficient, medical therapy and, in some cases, surgery might be considered (Fig. 13.2).
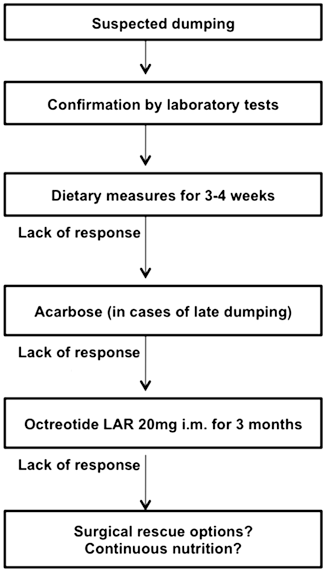

Fig. 13.2
Proposed treatment algorithm for dumping syndrome
Diet
The resolution of dumping symptoms is achieved in most cases by dietary modification, in particular by the reduction of carbohydrate intake, and lifestyle adjustment.
Dietary measures include advising patients to consume smaller amounts at one time by dividing the recommended daily energy intake between six meals. Dietary prohibitions are very important. Fluid intake during meals should be restricted. Drinking liquids should be avoided for at least one half-hour after a meal. Complex carbohydrates (e.g., unsweetened cereals, pasta, potatoes, fresh fruit, and vegetables) are preferred. All rapidly absorbable carbohydrates (e.g., all sweet or sweetened foods) should be eliminated from the diet to prevent late dumping symptoms. Protein (e.g., meat, fish, chicken, eggs) and fat intake should be increased to meet daily caloric needs because of the restricted intake of carbohydrates. Many patients modify their diet according to their personal experiences with food tolerance.
Most individuals with relatively mild symptoms will respond to dietary changes. For patients with severe vasomotor symptoms (postprandial hypotension), lying supine for 30 min after meals may minimize the chance of syncope by delaying gastric emptying and improving venous return. Supplementation of dietary fibers (bran, methylcellulose) with meals has been proven effective in the treatment of hypoglycemic episodes. Increasing the viscosity of food, which slows down gastric emptying, is another approach to improve dumping symptoms. Fifteen grams of guar gum or 5 g of pectin with each meal has been tested with good results, especially in the pediatric population [25, 26]. However, the palatability and tolerability of these supplements is poor. Moreover, these substances are usually not readily available as pharmaceutical products at sufficiently high doses.
Pharmacologic Therapy
In approximately 3–5 % of patients, severe dumping will continue despite dietary modifications. This results in marked weight loss, fear of eating and outdoor activities, or even an inability to maintain full-time employment. Drug therapy plays an important role in patients who failed dietary changes.
Acarbose
Acarbose is an α-glucosidase inhibitor that interferes with carbohydrate absorption in the small intestine. It is a generic drug sold in Europe and Asia as Glucobay (Bayer AG), in North America as Precose (Bayer Pharmaceuticals), and in Canada as Prandase (Bayer AG). Acarbose significantly blunts the postprandial rise of glucose and insulin by delaying carbohydrate digestion. Because of the reversible nature of the inhibitor–enzyme interaction, the conversion of complex carbohydrates (starch and sucrose) to monosaccharides is delayed rather than completely blocked. This mechanism is responsible for the effectiveness of acarbose in late dumping. The positive effects of acarbose have been documented after a test meal in a few studies (Table 13.2). In a double-blinded study of nine patients after gastric surgery, acarbose given at a dose of 50 mg following a normal carbohydrate-rich meal has been shown to reduce the symptoms of postprandial hypoglycemia, especially in combination with pectin [27]. A higher dose of acarbose (100 mg) has not been found to have any beneficial effect.
Table 13.2
Summary of studies that evaluated the effect of acarbose in dumping syndrome
Study | No. of patients | Treatment | Result |
|---|---|---|---|
McLoughlin et al. (1979) | 10 | Acarbose 100 mg; single administration before OGTT | Improved symptoms and glycemia during OGTT; reduced rise in plasma levels of GIP and insulin |
Gerard et al. (1983) | 24 | Acarbose 100 mg; single administration before OGTT | Improved glycemia during OGTT; reduced increase in plasma insulin level; inhibition of glucose-induced glucagon suppression |
Lyons et al. (1985) | 13 | Acarbose 50 mg; single administration before standard breakfast | Significant attenuation of hyperglycemia; reduced rise in plasma levels of GIP, enteroglucagon, and insulin; no influence on plasma levels of VIP and somatostatin; no significant effect on symptoms |
Hasegawa et al. (1998) | 6 | Acarbose 50–100 mg; 3 times daily before meals for a month | Attenuation of glucose fluctuations and improvement of dumping symptoms (uncontrolled) |
This treatment approach, however, affects only the symptoms of late dumping, owing to the mode of action of acarbose. In addition, acarbose treatment often results in bloating, flatulence, and diarrhea, as the unabsorbed carbohydrates undergo bacterial fermentation in the small intestine; despite the decrease in these symptoms with time, these adverse effects might hamper treatment compliance.
Somatostatin Analogs
Somatostatin and its analog octreotide (Sandostatin®) [28] cause decreases in several GI peptides (insulin, glucagon, VIP, GIP, neurotensin, etc.) that usually increase after meals. In addition, these compounds directly decrease gastric emptying and bowel motility, leading to decreased nutritional absorption and blood flow in the bowel [29]. As such, these analogs show a broad range of activity against the full spectrum of symptoms of dumping syndrome. Both fast-acting and delayed-release somatostatin analogs have been used in the treatment of dumping syndrome. Fast-acting or long-acting repeatable (LAR) formulations of octreotide are the agents that have been most commonly studied [14, 28, 30–35].
Studies of the Fast-Acting Somatostatin Analog Octreotide
The results of several short-term studies of subcutaneously administered octreotide have shown efficacy in improving symptoms, improving glycemia, and slowing gastric emptying (Table 13.3) [30–33]. However, the need for 3–4 daily injections is potentially a major limitation for the long-term application of fast-acting somatostatin analogs. Three studies have evaluated the long-term use of subcutaneously administered octreotide in the treatment of dumping syndrome. Geer et al. found that long-term octreotide therapy (15 months on average) provided sustained symptom control [32]. Out of ten patients, eight received three daily injections of 100 μg octreotide, which resulted in good symptom control; seven individuals were able to resume work. Similarly, Vecht et al. evaluated the long-term effect of three daily doses of 25–200 μg octreotide in 20 patients with a mean follow-up of 37 months [36]. All patients had an initial positive response; at 3 months, 80 % continued this positive response. After 10 years, however, 11 of the 20 patients had discontinued therapy for a variety of reasons, including a lack of effect at 3 months ( n = 4), diarrhea ( n = 4), painful injections ( n = 1), reversible alopecia ( n = 1), and weight loss ( n = 1). Similar data were obtained in a larger group of patients, in whom long-term effects seemed less favorable than short-term effects, although 41 % of the cohort continued octreotide therapy after the follow-up period of 93 ± 15 months [34].




Table 13.3
Summary of studies that evaluated the effect of octreotide in dumping syndrome
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





