Cytology of Renal and Adrenal Lesions
Jasreman Dhillon
Debra L. Zynger
Marino E. Leon
Percutaneous sampling of renal masses has increased with the recent advances in interventional radiology techniques. Tissue from renal and adrenal masses can be sampled either by fine needle aspiration (FNA) or by thin core needle biopsy. The advantages of FNA are that it is relatively less invasive, cost-effective, amenable to immediate assessment, and can guide the radiologist regarding accurate targeting of the lesion for therapeutic intervention as well as for assessment of specimen adequacy. The cytology specimens may be collected by percutaneous computed tomography (CT) or ultrasound guidance, or by endoscopic ultrasound guidance through a gastrointestinal approach (1,2). However, it should be stated that core needle biopsy provides distinct advantages including preservation of the architecture of the tumor and better subclassification. Additionally, core needle biopsy provides a reserve of histologic tissue for ancillary techniques if needed.
Rapid on-site evaluation of the cytology specimen at the time material is obtained allows for a timely communication with the radiologist or physician performing the procedure. An appropriate triage of the material may be performed at this time. The need for ancillary studies for diagnosis or guiding personalized therapies may require extra passes to obtain additional material for an adequate cell block, or a needle core biopsy may be needed and performed. Furthermore, the rapid on-site evaluation of delicate touch preparations of the obtained core biopsies by trained cytopathologists and cytotechnologists allows for the quick assessment of adequacy of the needle core biopsies. These steps may avoid an unsatisfactory result and the need for repeat procedures.
Immunohistochemical stains are usually performed on sections of the cell blocks; it is preferable to stain sections from a cell block rather than smears as laboratories may use routine immunohistochemical methods and controls. Pathologists are more familiar with interpreting immunostained tissue sections than stained smears. Pathologists should be careful about not wasting this precious material that may also be required
for ancillary molecular studies (e.g., EGFR mutation, ALK translocation). As immunostains are very likely to be needed, unstained slides may be obtained during the preparation of routine hematoxylin and eosin sections, minimizing the loss of tissue.
for ancillary molecular studies (e.g., EGFR mutation, ALK translocation). As immunostains are very likely to be needed, unstained slides may be obtained during the preparation of routine hematoxylin and eosin sections, minimizing the loss of tissue.
FINE NEEDLE ASPIRATION CYTOLOGY OF RENAL LESIONS
Normal Elements
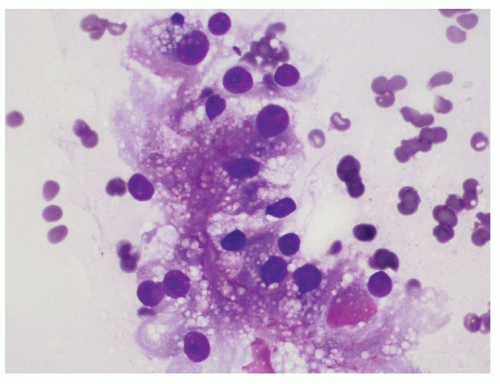
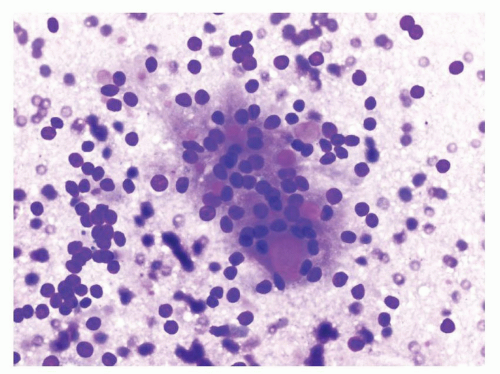
Normal elements aspirated from the renal parenchyma include the proximal and distal tubular epithelial cells and glomeruli. The proximal tubular epithelial cells are present in loose aggregates which may show tubule formation. These cells have abundant granular cytoplasm and a single round nucleus and may resemble tumor cells of an oncocytoma and chromophobe renal cell carcinoma (RCC). The distal tubular epithelial cells have relatively scant granular cytoplasm as compared to the proximal tubules. These cells can be confused with clear cell RCC and papillary RCC. The glomeruli are large dense round structures which contain capillary loops within them. Glomeruli can be confused with RCC on smears (Fig. 14.1).
Clear Cell Renal Cell Carcinoma
The cytology smears are usually very hemorrhagic due to the rich vascularity of the tumor. The neoplastic cells are present in large cohesive cell
groups and as single cells. The malignant cells have abundant cytoplasm and a single round nucleus with a prominent nucleolus (Fig. 14.2). The nuclear to cytoplasmic ratio is low. The cytoplasm is translucent and may be vacuolated (Fig. 14.3). Cell membranes are poorly defined. In higher grade tumors, more isolated single cells are present. The cytoplasm becomes less vacuolated and the nucleoli are more prominent and larger. Neutrophils may be present within the cytoplasm of the tumor cells (eFig. 14.1). In low-grade tumors, the neoplastic cells are arranged in small sheets which resemble the normal distal tubular epithelial cells. Apart from the increased cellularity in the smears from tumors, the cytomorphology of the tumor cells can be very similar to the normal distal tubular epithelial cells (3,4). Cell block material is very helpful in these situations (eFig. 14.2).
groups and as single cells. The malignant cells have abundant cytoplasm and a single round nucleus with a prominent nucleolus (Fig. 14.2). The nuclear to cytoplasmic ratio is low. The cytoplasm is translucent and may be vacuolated (Fig. 14.3). Cell membranes are poorly defined. In higher grade tumors, more isolated single cells are present. The cytoplasm becomes less vacuolated and the nucleoli are more prominent and larger. Neutrophils may be present within the cytoplasm of the tumor cells (eFig. 14.1). In low-grade tumors, the neoplastic cells are arranged in small sheets which resemble the normal distal tubular epithelial cells. Apart from the increased cellularity in the smears from tumors, the cytomorphology of the tumor cells can be very similar to the normal distal tubular epithelial cells (3,4). Cell block material is very helpful in these situations (eFig. 14.2).
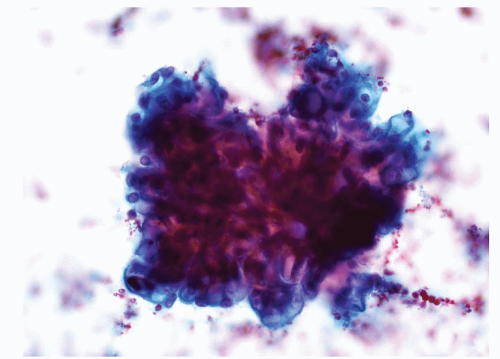 FIGURE 14.1 Glomerulus: a round structure with small-sized capillaries evident toward the periphery (Papanicolaou stain). |
 FIGURE 14.2 Clear cell RCC: a cluster of cells with round nuclei, prominent nucleoli, and a moderate amount of cytoplasm with indistinct cell membranes (Papanicolaou stain). |
The differential diagnosis includes benign tubular epithelial cells, adrenal cortical cells, hepatocytes, macrophages, and other subtypes of RCC such as papillary RCC and MiTF family associated RCC. Aspirates of benign tubular epithelial cells are hypocellular and are present in small clusters. These cells usually lack vacuolated cytoplasm. Adrenal cortical cells are composed of bare nuclei without significant atypia, often present in a background of proteinaceous debris. The cytomorphologic features of papillary RCC and translocation RCC are described in detail in Chapters 3 and 4, respectively. Immunohistochemical stains are very helpful in differentiating clear cell RCC (positive for carbonic anhydrase IX [CA-IX], CD10, and epithelial membrane antigen [EMA]; negative for
CK7 and AMACR) from papillary RCC (positive for CD10, EMA, CK7, and AMACR; negative or focally positive for CA-IX) or translocation RCC (positive for CD10 and AMACR; negative for CK7 and EMA). In addition, translocation RCC will be positive for the immunohistochemical stain TFE3.
CK7 and AMACR) from papillary RCC (positive for CD10, EMA, CK7, and AMACR; negative or focally positive for CA-IX) or translocation RCC (positive for CD10 and AMACR; negative for CK7 and EMA). In addition, translocation RCC will be positive for the immunohistochemical stain TFE3.
Papillary Renal Cell Carcinoma
On smears, the cells of papillary RCC may be arranged in papillae containing fibrovascular cores, spherules, and tubules (Figs. 14.4 and 14.5, eFig. 14.3). The background can show abundant macrophages or may be necrotic (eFig. 14.4). The fibrovascular cores may be distended with macrophages. The lining epithelial cells of type 1 papillary RCC are usually round and monomorphic. Type 2 nuclei are more atypical and have prominent nucleoli (Fig. 14.6). Intracytoplasmic hemosiderin deposition and psammoma bodies may be admixed with the tumor cells (5,6,7,8). Immunohistochemistry may be helpful for the diagnosis. At the same time, diagnosis of type 2 papillary RCC can only be suggested and should not be made with certainty on FNA material, as a number of other primary renal tumors can show similar cytoarchitectural features in limited material.
Normal renal parenchymal components such as glomeruli and distal tubular epithelial cells can be confused with papillary RCC on smears. The cytomorphology of papillary RCC type 1 is similar to that of distal tubular epithelial cells. However, the aspirations containing distal tubular epithelial
cells are paucicellular and the cells are not arranged in papillae and spherules. On low power, glomeruli may appear as spherules, but on higher power examination, the capillary loops present within the glomeruli help in recognizing them. Metanephric adenoma may be extremely difficult to distinguish from papillary RCC, especially type 1, on smears alone. Immunohistochemical stains on a cell block may help distinguish papillary RCC (positive for CK7 and EMA; negative for WT1) from metanephric adenoma (positive for WT1 and CD57; negative for CK7 and EMA). Clear cell RCC and translocation RCC are also included in the differential diagnosis.
cells are paucicellular and the cells are not arranged in papillae and spherules. On low power, glomeruli may appear as spherules, but on higher power examination, the capillary loops present within the glomeruli help in recognizing them. Metanephric adenoma may be extremely difficult to distinguish from papillary RCC, especially type 1, on smears alone. Immunohistochemical stains on a cell block may help distinguish papillary RCC (positive for CK7 and EMA; negative for WT1) from metanephric adenoma (positive for WT1 and CD57; negative for CK7 and EMA). Clear cell RCC and translocation RCC are also included in the differential diagnosis.
Chromophobe Renal Cell Carcinoma
The aspirates are very cellular and show loosely cohesive tumor cells that have abundant granular and fluffy cytoplasm in the classical chromophobe RCC (eFig. 14.5). Nuclei are pleomorphic with the presence of binucleation, irregular nuclear contours, grooves, and pseudoinclusions. Nucleoli are usually not prominent. Perinuclear clearing is best appreciated on the Romanowsky-stained smears. These cytomorphologic findings when fully developed give the tumor cells a koilocytoid appearance which is very characteristic of chromophobe RCC (Fig. 14.7) (9,10). The eosinophilic variant is composed of cells that are smaller than the cells of the classic variant and have lesser volume of eosinophilic cytoplasm. These features make it very difficult to distinguish them from oncocytoma.
 FIGURE 14.7 Chromophobe RCC: a binucleated tumor cell exhibiting mild pleomorphism (Diff-Quik stain). |
Classic variant of chromophobe RCC should be distinguished from clear cell RCC and the eosinophilic variant from oncocytoma. The nuclei of clear cell RCC are round with prominent nucleoli, whereas chromophobe RCC nuclei are irregular, hyperchromatic, and may not have prominent nucleoli. Perinuclear halos when present are helpful to render a diagnosis of chromophobe RCC. Nuclei of an oncocytoma are round with conspicuous small nucleoli. When in doubt, a diagnosis of an oncocytic neoplasm can be rendered with a differential diagnosis of eosinophilic chromophobe RCC and oncocytoma. Chromophobe RCC is usually diffusely and strongly positive for CK7, whereas oncocytoma is negative or only focally positive. However, some eosinophilic chromophobe RCCs may show very focal staining for CK7.
MiTF Family Associated RCC
On cytology smears, the tumor cells may form papillae and spherules (eFig. 14.6). The tumor cells have abundant translucent cytoplasm and a single round nucleus with a prominent nucleolus (Fig. 14.8). Alternatively, the tumor cells may be arranged around small acellular hyaline material (Fig. 14.9), a feature that is more commonly seen in TFE3 tumors. Psammoma bodies may be present in the background. Nuclear positivity for TFE3 immunostain is a reliable marker for this RCC. Clear cell RCC and papillary RCC are in the differential diagnosis. Immunohistochemical stains on cell block sections help diagnose this relatively rare entity.
 FIGURE 14.8 Translocation associated RCC: tumor cells have a moderate amount of cytoplasm and a round nucleus with a conspicuous nucleolus (Papanicolaou stain). |
Medullary Renal Cell Carcinoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree