Fig. 7.1
Proposed metabolic pathway of 5-ASA after oral administration. The shaded area (large intestine) indicates the site of topical action. Unformulated 5-ASA is absorbed rapidly from the small intestine, and many current formulations are designed to delay the release of 5-ASA until the terminal ileum or proximal colon. 5-ASA 5-aminosalicylic acid, N-AC-5-ASA N-acetyl-5-ASA (Reprinted from Lichtenstein GR, Kamm MA. Review article: 5-aminosalicylate formulations for the treatment of ulcerative colitis- methods of comparing release rates and delivery of 5-aminosalicylate to the colonic mucosa. Alimentary Pharmacol Ther 2008; 28 (6): 663-73; Copyright 2008) [73]
The colon epithelial cells absorb mesalamine released in the colon. In the epithelial cells, N-acetyl transferase 1 (NAT-1) enzyme metabolizes mesalamine or 5-ASA into N-Acetyl-5ASA. This is either secreted back into the lumen and excreted in feces or absorbed into the circulation and excreted in the urine [14]. Some of the 5-ASA is absorbed into the systemic circulation and undergoes acetylation in the liver and is excreted in the urine [14]. A very small portion of 5-ASA undergoes acetylation by colon bacteria and excreted in the feces [14].
Are there any differences in the pharmacokinetic profiles of various oral mesalamine preparations? A recent systematic review by Sandborn and Hanauer showed that the systemic absorption of mesalamine is comparable for all oral preparations. They demonstrated that the urinary and fecal excretion of total 5-ASA was similar among all oral mesalamine preparations [14]. This was also confirmed by a study comparing pharmacokinetic profiles of equimolar doses of Asacol and balsalazide [15].
Mesalamine Formulations and Preparations
A variety of mesalamine formulations have been developed that ensure maximal delivery of active 5-ASA to the terminal ileum and colon. These include both oral and topical mesalamine preparations. While oral preparations ensure maximal 5-ASA concentrations in the proximal colon, topical preparations deliver 5-ASA directly to the left side of the colon. Tables 7.1 and 7.2 summarize various oral and topical 5-ASA preparations currently available, respectively.
Table 7.1
5-ASA formulations and release sites
Name | Formulation/release site | Dosage |
|---|---|---|
Sulfasalazine/Azulfidine® | 5-ASA linked to sulfapyridine by an azo bond | 500 mg tablets |
Asacol®/delayed-release mesalamine | Enclosed in enteric “film” (Eudragit-S) releasing at pH ≥ 7 in the terminal ileum and colon | Asacol, 400 mg |
Asacol HD, 800 mg | ||
Salofalk®, Claversal® | Enclosed Eudragit-L releasing at pH ≥ 6 in the jejunum, ileum, and colon | 250 and 500 mg tablets |
Pentasa® | Microspheres within a moisture-sensitive, ethyl cellulose, semipermeable membrane releasing mesalamine in the duodenum, jejunum, ileum, and colon | 250 and 500 mg capsules |
Apriso® | The outer coating (Eudragit-L) dissolves in the jejunum, ileum, and colon (pH ≥ 6), while a polymer matrix core facilitates slow, sustained release throughout the colon | 375 mg |
Olsalazine/Dipentum® | Two molecules of 5-ASA linked by an azo bond between their amino groups cleaved by azoreductase | 250 mg |
Balsalazide disodium/Colazal® | 5-ASA linked by an azo bond to 4-amino-benzoyl-β-alanine cleaved by azoreductase | 750 mg |
Lialda®, Mezavant® | Has lipophilic and hydrophilic matrices to provide delayed release of mesalamine. Has Eudragit-S which enables mesalamine release at pH ≥ 7 in the terminal ileum and continues throughout the colon | 1.2 g |
Salofalk Granu-Stix® and Pentasa® sachets | Micropellet formulations | 500 mg sachets |
Table 7.2
Topical 5-ASA preparations
Formulation | Brand name | Dosages |
|---|---|---|
Suspension enema | Rowasa, Asacol, Pentasa, Salofalk | 1 g/60 ml, 2 g/60 ml, 4 g/60 ml |
Suppository | Canasa, Asacol, Claversal, Pentasa | 250, 400, 500, 100 mg |
Gel | Asacol, Claversal, Salofalk | 1 g/30 ml, 1 g/60 ml, 2 g/60 ml, 2 g/120 ml |
Foam | Enterasin | 2 g/60 ml |
Oral pH-Dependent Formulations
These include Asacol, Ipocol, Claversal, Salofalk, Apriso, and Lialda. In order to prevent proximal absorption, mesalamine is coated with pH-sensitive polymers. The two most common polymers used are Eudragit-S and Eudragit-L [8, 12].
Eudragit-S coating breaks down at pH > 7 and releases mesalamine in the terminal ileum and colon [12]. Asacol (Procter and Gamble Pharmaceuticals, Cincinnati, OH, USA) and Ipocol (Sandoz Pharmaceuticals, Bordon, Hampshire, UK) are examples of mesalamine preparations which are enteric coated with Eudragit-S [8, 12, 16]. Asacol is available as 400 and 800 mg tablets and administered 2–3 times per day [17]. Eudragit-L, a derivative of S-polymer, breaks down at a lower pH and releases mesalamine in the jejunum, terminal ileum, and colon [12]. Claversal (Merckle GMBH, Ulm, Germany) and Salofalk (Axcan Pharma, Mont St. Hilaire, Quebec, Canada, and Falk Pharma, Freiburg, Germany) are examples of mesalamine formulations coated with Eudragit-L [12]. Studies on healthy volunteers and ileostomy patients have demonstrated that Eudragit-L-coated mesalamine preparations are released more proximally than Eudragit-S-coated mesalamine preparations [12].
Apriso (Salix Pharmaceuticals Inc., Morrisville, NC, USA) has been approved by FDA only for the maintenance of remission in ulcerative colitis [8]. It consists of a gelatin capsule containing granules of mesalamine, which are coated with Eudragit-L polymer resin. The capsule dissolves in the stomach and releases the mesalamine granules which in turn break down at pH greater than 6. The granules also contain a polymer matrix, which swells and ensures gradual release of the mesalamine throughout the colon [8]. It is dosed once daily.
MMX mesalamine marketed as Lialda in the USA and Mezavant elsewhere is a novel pH-dependent once-daily mesalamine formulation [8, 12, 16]. FDA has approved it for both induction and maintenance of remission in ulcerative colitis patients. In this formulation, MMX technology is utilized. It contains both hydrophilic and lipophilic matrices, which are in turn coated by pH-sensitive Eudragit-S resin. The Eudragit-S coating delays release of mesalamine until the terminal ileum and colon where pH is greater than 7. The hydrophilic matrix then comes in contact with intestinal fluids and swells forming a viscous gel mass [12, 16]. The viscous gel mass ensures slow release of the mesalamine. The lipophilic core prevents the water from entering the core of the tablet and dissolving it. This helps to prolong the half-life of the drug [8, 12, 16]. MMX mesalamine is available as 1,200 mg tablets and administered once or twice daily [17].
Oral pH-Independent Formulations
This includes Pentasa (Shire Pharmaceuticals Inc., Wayne, PA, USA, licensed from Ferring A/S Copenhagen, Denmark), a controlled-release mesalamine formulation. It utilizes a semipermeable, moisture-sensitive ethyl cellulose coating [16]. This allows a slow and sustained release of mesalamine. It also differs from other mesalamine preparations by releasing mesalamine in the duodenum and jejunum, in addition to the terminal ileum. It is estimated that approximately about 20 % of mesalamine is released in the small intestine and the rest in the colon [12]. It is available as 250 and 500 mg tablets and 250 mg capsules. It is administered four times per day.
Both Pentasa and Salofalk are available as sachets (micropellet formulations). One of the advantages of micropellet formulations is that it facilitates prolonged release of the mesalamine, thereby allowing less frequent dosing. This has been demonstrated in two studies comparing tablets to micropellets [16].
Mesalamine Prodrug Formulations
These mesalamine formulations similar to sulfasalazine utilize a diazo bond (Fig. 7.2). The diazo bond is cleaved by colon bacteria’s azoreductase enzyme-releasing mesalamine. Balsalazide and olsalazine are examples of prodrugs. Balsalazide/Colazal (Salix Pharmaceutical Inc., Morrisville, NC) utilizes an inert molecule (4-aminobenzoyl-β-alanine) for binding with a single 5-ASA molecule. Balsalazide is available as 750 mg tablets and is administered three times per day [17]. Olsalazine (Alaven Pharmaceuticals, Marietta, GA/Dipentum (UCB Pharma, Brussels, Belgium)) contains two 5-ASA molecules linked by a diazo bond. Olsalazine is available as 250 mg capsules and is administered twice daily [17].
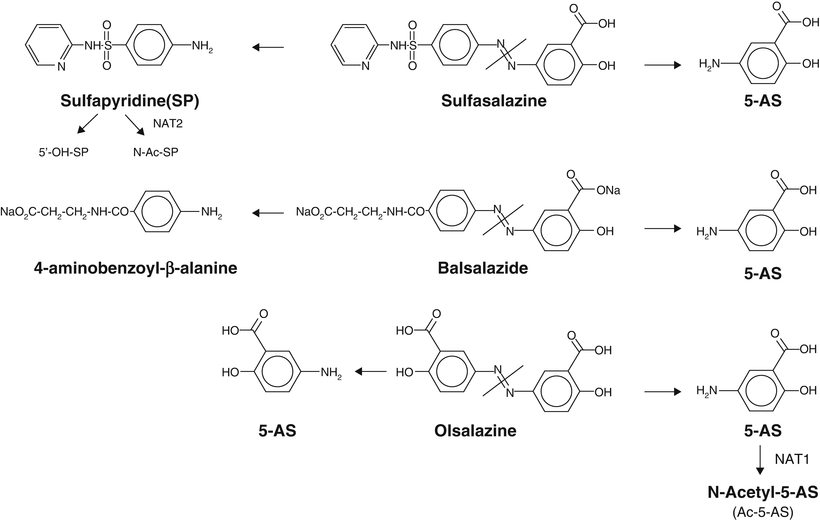

Fig. 7.2
Structure of different prodrugs of 5-AS and part of their metabolism. Reprint from Klotz U, Schwab M. Topical delivery of therapeutic agents in the treatment of inflammatory bowel disease. Adv Drug Delivery Rev 2005;57:267-79; © Elsevier 2005) [74]
Topical Mesalamine Formulations
Topical mesalamine preparations deliver 5-ASA directly to the site of inflammation in the distal colon. They are available as suppositories, suspension enemas, gels, and foams. Currently in the USA, only mesalamine suppositories (Canasa) and suspension enemas (Rowasa) are available. Scintigraphy studies have demonstrated that suppositories deliver active drug to the rectum, while suspension enemas can reach as far as the splenic flexure [12].
Efficacy of Mesalamine in the Induction of Remission in Active UC
The first placebo-controlled study demonstrating sulfasalazine’s efficacy in the induction of remission in UC was published by Baron et al. [2]. But due to side effects and intolerance of sulfasalazine, newer mesalamine preparations have been developed in an effort to reduce side effects and improve efficacy. In this section, we will discuss how newer mesalamine preparations compare to placebo and sulfasalazine in the induction of remission and also evaluate if there are any differences among various mesalamine preparations in their ability to induce remission. It is important to recognize that different studies used varied definitions for clinical and endoscopic remissions making comparison between various 5-ASA preparations difficult. Refer to Table 7.3 for randomized double-blind controlled trials comparing therapy with various oral 5-ASA formulations in inducing remission of UC.
Table 7.3
Randomized double-blind controlled trials comparing therapy with various oral 5-ASA formulations in inducing remission of ulcerative colitis
Reference | Treatment arm | Daily dose | # patients | Study duration | Primary end point | Results | AEs | Withdrawals | Comments |
|---|---|---|---|---|---|---|---|---|---|
Fleig et al. [75] | Sulfasalazine | 3.0 g | 21 | 6 w | Improvement in mean stool frequency (A) and consistency (B) | A: 6.9 ± 3.4 vs. 3.0 ± 1.9 (p < 0.05) | Nausea (3 p) | Side effects (generalized exanthema) (1 p) | No statistical difference in efficacy between sulfasalazine and benzalazine changes in macroscopic and microscopic appearance of the colonic mucosa |
Pruritus (1 p) | |||||||||
Generalized exanthema (1 p) | |||||||||
B: Significant improvement | |||||||||
C: Improvement 8 p | |||||||||
No change: 8 p | |||||||||
Worsening: 0 p | |||||||||
D: improvement: 8 p | |||||||||
No change: 7 p | |||||||||
Worsening: 2p | |||||||||
Macroscopic (C) and microscopic (D) appearance of the colonic mucosa measured at w 0 and w 6 | |||||||||
Benzalazine | 2.16 g | 22 | A: 6.8 ± 2.1 vs. 4.0 ± 2.7 (p < 0.05) | 3 p: nausea and vomiting | Rapid worsening of disease (3 p) | ||||
B: significant improvement | Lost to follow-up (2 p) | ||||||||
C: Improvement 11 p | |||||||||
No change: 5 p | |||||||||
Worsening: 0 p | |||||||||
D: Improvement: 8 p | |||||||||
No change: 7 p | |||||||||
Worsening: 0 pts | |||||||||
Riley et al. [76] | Sulfasalazine | 2 g | 19 | 4 w | Improvement in stool frequency, rectal bleeding, and macroscopic and microscopic grade of the colonic mucosa measured at w 0 and w 4 | Significant improvement of macroscopic score above 5 cm at w 4 (p < 0.005) | Itchy rash (2 p) | Itchy rash (2 p) | Improvement in rectal bleeding and macroscopic grade of the colonic mucosa at w 4 was significantly greater in pts treated with high-dose mesalazine than sulfasalazine (p < 0.05) |
Headache (6 p) | |||||||||
GI symptoms (anorexia, nausea, vomiting, dyspepsia) (4 p) | |||||||||
Mesalamine | 0.8 g | 20 | Significant improvement of rectal bleeding (p < 0.005), macroscopic (p < 0.01) and microscopic (p < 0.005) scores at w 4 | Headache (4 p) | |||||
GI symptoms (anorexia, nausea, vomiting, dyspepsia) (4 p) | |||||||||
Mesalamine | 2.4 g | 21 | Significant improvement of stool frequency, (p < 0.01), rectal bleeding (p < 0.01), and macroscopic score (p < 0.005) at w 4 | Headache (5 p) | Up to twofold increase in plasma creatinine (2 p) | ||||
GI symptoms (anorexia, nausea, vomiting, dyspepsia) (7 p) | |||||||||
Rachmilewitz [77] | Coated mesalamine | 1.5 g | 115 | 8 w | Clinical and endoscopic remission: clinical and endoscopic activity score ≤4 | Clinical remission: | 16/115 p (14 %) | 7/115 p (6 %) | 164 patients were included in the efficacy analysis (87 received coated mesalazine and 77 received sulfasalazine) |
w 4: 50/70 pts (71 %) | |||||||||
w 8: 37/50 p (74 %) | |||||||||
Endoscopic remission: | |||||||||
w 8: 20/41 pts (49 %) | |||||||||
Total number of AE: 29 | |||||||||
Sulfasalazine | 3 g | 105 | Clinical remission: | 25/105 p (24 %) | 8/105 p (8 %) | ||||
w 4: 38/58 p (66 %) (p = 0.338 vs. coated mesalazine) | |||||||||
w 8: 35/43 p (81 %) (p = 0.835 vs. coated mesalazine) | |||||||||
Endoscopic remission: | |||||||||
w 8: 18/38 p (47 %) (p = 0.272 vs. coated mesalazine) | |||||||||
Total number of adverse events: 47 | |||||||||
Rao et al. [78] | Olsalazine | 2 g | 20 | 4 w | Overall improvement defined as a positive change in at least two of the following criteria: | Overall improvement at w 4 vs. w 0 | Headache and nasal stuffiness (1 p) | 2 | No difference in the overall response between treatment arms |
Diarrhea (1 p) | |||||||||
Clinical activity index by Truelove and Witts % of bloody stools | |||||||||
Significantly greater decrease in proportion of unformed stools at w 4 in patients treated with sulfasalazine vs. olsalazine (p < 0.05) | |||||||||
No difference in tolerance between treatment arms | |||||||||
Sigmoidoscopic appearance of the colon | |||||||||
Histologic appearance of the colonic mucosa | |||||||||
15/18 p (83 %) (p < 0.01) | |||||||||
Proportion of unformed stools (78 % at w 0 vs. 55 % at w 4, p < 0.001) | |||||||||
Bloody stools (61 % at w 0 vs. 22 % at w 4, p < 0.001) | |||||||||
Improvement in sigmoidoscopic score at w 4 vs. w 0: 83 % (p < 0.01) | |||||||||
Improvement in histologic score at w 4 vs. w 0: 44 % (p < 0.01) | |||||||||
Sulfasalazine | 3 g | 17 | Overall improvement at w 4 vs. w 0 | Dyspepsia and nausea (2 p) | 4 | ||||
9/13 p (69 %) (p < 0.01) | Exacerbation of bloody diarrhea (1 p) | ||||||||
Proportion of unformed stools (72 % at w 0 vs. 28 % at w 4, p < 0.001) | |||||||||
Bloody stools (67 % at w 0 vs. 37 % at w 4, p < 0.001) | |||||||||
Improvement in sigmoidoscopic score at w 4 vs. w 0: 84 % (p < 0.01) | |||||||||
Improvement in histologic score at w 4 vs. w 0: 46 % (p < 0.01) | |||||||||
Myalgia, headache, and dizziness (1) | |||||||||
Munakata et al. [79] | Mesalamine | 1.5 g | 52 | 4 w | Improvement in clinical symptoms and endoscopic findings | Marked and moderate clinical improvement: | 6/52 p (11.5 %) | Not reported | No difference in clinical and endoscopic improvement between treatment arms |
30/48 p (63 %) | |||||||||
Sulfasalazine | 3.0 g | 57 | Marked and moderate clinical improvement: | 16/57 p (28.1 %) | Not reported | ||||
General usefulness based on the improvement and safety: | |||||||||
32/52 p (62 %) | |||||||||
mesalazine 65.3 % vs. sulfasalazine 45.6 % (p = 0.042) | |||||||||
Kruis et al. 1998 [31] | Olsalazine | 3 g | 88 | 12 w | Endoscopic remission: score 0 or 1 on 5-point scale | 52.2 % | 41/88 p (46 %) | 11/88 p (13 %) | – |
Mesalamine | 3 g | 80 | 48.8 % (p = 0.67, vs. olsalazine) | 29/80 p (36 %) | 9/80 p (11 %) | ||||
Score 0: normal mucosa with visible vascular pattern, no granularity or friability | |||||||||
Score 1: inactive colitis, pink mucosa, no visible blood vessels, faintly granular but no friability | |||||||||
Green et al. [80] | Balsalazide | 6.75 g | 50 | 12 w | Complete remission at w 4, 8, and 12: symptomatic remission with no use of relief medication in the previous 4 days and grade 0 or 1 on sigmoidoscopy | w 4: 38 % | 24/50 p (48 %) | 15/50 p (30 %) | – |
Treatment failure 6/50 p (12 %) | |||||||||
w 8: 54 % | AE: 1/50 p (2 %) | ||||||||
w 12: 62 % | |||||||||
Mesalamine | 2.4 g | 49 | w 4: 12 % | 35/49 p (71 %) | 23/49 p (47 %) | ||||
[Grade 0: normal, vascular pattern clearly visible | |||||||||
p = 0.024 | Treatment failure 16/49 p (33 %) | ||||||||
w 8: 22 % | |||||||||
w 12: 37 % | |||||||||
AE: 1/49 p (2 %) | |||||||||
Grade 1: erythema with loss of vascular pattern] | |||||||||
p < 0.01 | |||||||||
p = 0.068 | |||||||||
p < 0.01 | |||||||||
p = 0.015 | |||||||||
p < 0.05 | |||||||||
Green et al. [111] | Balsalazide | 6.75 g | 28 | 12 w | Remission rates at the end of study or withdrawal/remission: return to stool frequency (with or without pain) to that before relapse without the presence of blood and confirmed by biopsy | Completed study in remission: 21/28 p (75 %) | Serious: 2/28 pts (7 %) | AE: 2/28 pts (7 %) | |
Minor: 27/28 pts (96 %) | Treatment failure: 1/28 p (4 %) | ||||||||
Lost to follow-up: 0/28 (0 %) | |||||||||
Sulfasalazine | 3 g | 29 | Completed study in remission: 17/29 p (69 %) | Serious: 0/29 p (0 %) | AE: 9/29 p (31 %) | ||||
Minor: 27/29 p (93 %) | Treatment failure: 1/29 p (3 %) | ||||||||
p = 0.19 | |||||||||
Lost to follow-up: 1/29 (3 %) | |||||||||
p = 0.041 | |||||||||
p > 0.2 | |||||||||
p > 0.2 | |||||||||
Forbes et al. [82] | Asacol-mesalamine in Eudragit-S coating | 2.4 g | 42 | 8 w | Efficacy based on modified St. Mark’s Colitis Activity Score, macroscopic and microscopic appearance of the rectum and (PGA). Clinical remission defined from PGA | Decrease in St. Mark’s Colitis Activity Score: −2.3 | 31/42 p (73.8 %) | 11/42 p (26.1 %) | – |
Clinical remission w 8: 28.6 % | |||||||||
Improvement in sigmoidoscopy score: 54.8 % | |||||||||
Improvement in histologic score: 31 % | |||||||||
Ipocol-mesalamine in Eudragit-S coating | 2.4 g | 46 | Decrease in St. Mark’s Colitis Activity Score: −1.5 | 34/46 p (73.9 %) | 9/46 p (19.6 %) | ||||
Clinical remission w 8: 26.1 % | |||||||||
Improvement in sigmoidoscopy score: 50.0 % | |||||||||
Improvement in histologic score: 30.4 % | |||||||||
p = ns | |||||||||
p = ns | |||||||||
p = ns | |||||||||
p = ns | |||||||||
Pruitt et al. [83] | Balsalazide | 6.75 g | 84 | 8 w | Symptomatic remission: patient functional assessment ratings of normal or mild and absence of rectal bleeding at wk 8 or early completion of treatment | 38/73 p (52 %) | 45/84 p (54 %) | AE: 3/84 p (4 %) | 73 p in efficacy-evaluable population |
84 p in intention to treat population | |||||||||
39/84 p (46 %) | |||||||||
Mesalamine | 2.4 g | 89 | 38/77 p (49 %) | 57/89 p (64 %) | AE: 6/89 p (7 %) | 77 p in efficacy-evaluable population | |||
38/89 p (44 %) | 89 p in intention to treat population | ||||||||
Levine at al. [84] | Balsalazide | 6.75 g | 53 | 8 w | Improvement in rectal bleeding and in at least one other sign or symptom at w 8 | Improvement in rectal bleeding: 65 %, | 23/53 p (43 %) | 16/53 p (30 %) | 49 p in efficacy-evaluable population |
Improvement in stool frequency: 59 % | |||||||||
53 p in intention to treat population | |||||||||
Improvement in sigmoidoscopic score: 79 %, | |||||||||
Improvement in PGA: 74 %, | |||||||||
Improvement in overall symptom assessment: 65 % | |||||||||
Improvement in patient functional assessment: 71 % | |||||||||
Balsalazide | 2.25 g | 50 | Improvement in rectal bleeding: 32 % | 27/50 p (54 %) | 17/50 p (34 %) | 49 p in efficacy-evaluable population | |||
50 p in intention to treat population | |||||||||
Improvement in stool frequency: 29 % | |||||||||
Improvement in sigmoidoscopic score: 53 % | |||||||||
Improvement in PGA: 51 %, | |||||||||
p = 0.006 | |||||||||
p = 0.006 | |||||||||
p = 0.015 | |||||||||
p = 0.030 | |||||||||
Mesalamine | 2.4 g | 51 | Improvement in rectal bleeding: 53 % | 26/51 p (51 %) | 15/51 p (29 %) | 49 p in efficacy-evaluable population | |||
51 p in intention to treat population | |||||||||
Improvement in sigmoidoscopic score: 61 % | |||||||||
Improvement in PGA: 62 % | |||||||||
Improvement in overall symptom assessment: 58 % | |||||||||
Improvement in patient functional assessment: 61 % | |||||||||
p = ns | |||||||||
p = ns | |||||||||
p = ns | |||||||||
p = ns | |||||||||
p = ns | |||||||||
Mansfield et al. [85] | Balsalazide | 6.75 g | 26 | 8 w | Remission defined as stool frequency of ≤ 2/day without blood and with normal colonic mucosa or minimal erythema on sigmoidoscopy at w 8 | 13/26 p (50 %) | 17/26 p (65 %) | AE: 1/26 p (4 %) | |
Treatment ineffective: 2/26 p (7.5 %) | |||||||||
Protocol violation: 2/26 pts (7.5 %) | |||||||||
Sulfasalazine | 3 g | 24 | 9/24 p (38 %) | 21/24 p (88 %) | AE: 9/24 p (38 %) | ||||
Treatment ineffective: 3/24 pt (12 %) | |||||||||
Protocol violation: 1/24 p (4 %) | |||||||||
p = 0.10 | p = 0.004 | ||||||||
Raedler et al. [86] | Mesalamine micropellets | 3 g | 181 | 8 w | Clinical remission: clinical activity index according to Rachmilewitz ≤2 at w 8 | 67 % (intention to treat population) | 56/181 p (30.9 %) | Not reported | 179 p in intention to treat population |
64.4 % (according to protocol population) | 160 p in according to protocol population | ||||||||
181 p in safety population | |||||||||
Mesalamine tablets | 3 g | 181 | 62.9 % (intention to treat population) | 43/181 p (23.8 %) | Not reported | 178 p in intention to treat population | |||
64.2 % (according to protocol population) | |||||||||
OR = 1.199 (95%CI 0.758–1.897) | |||||||||
OR = 1.008 (95 % CI 0.623–1.632) | p = 0.43 | ||||||||
162 p in according to protocol population | |||||||||
181 p in safety population | |||||||||
Tursi et al. [87] | Balsalazide + VSL # 3 | 2.25 g + 3 g VSL #3 | 30 | 8 w | Symptomatic remission: patient functional assessment of normal bowel movements and absence of rectal bleeding | 24/30 p (80 %) (95 % CI 59–91) | Not reported | Protocol violation: 1/30 p (3 %) | |
Protocol ineffectiveness: 1/30 p (3 %) | |||||||||
Balsalazide | 4.5 g | 30 | 21/30 p (70 %) (95 % CI 43–81) | Not reported | Protocol violation: 1/30 p (3 %) | ||||
Protocol ineffectiveness: 3/30 p (10 %) | |||||||||
Mesalamine | 2.4 g | 30 | 16/30 p (53.3 %) (95%CI 42–62) | Not reported | Protocol violation: 2/30 p (6 %) | ||||
p < 0.02 | |||||||||
Protocol ineffectiveness: 4/30 p (13 %) | |||||||||
AE: 2/30 p (6 %) | |||||||||
Jiang and Cui [88] | Olsalazine | 1 g | 21 | 8 w | Complete remission: decrease in clinical symptoms with relative normal appearance of the colonic mucosa | Complete remission: 16/21 p (76 %) | Not reported | Not reported | – |
Sulfasalazine | 1 g | 21 | Complete remission: 10/21 p (48 %) | Not reported | Not reported | ||||
p < 0.05 | |||||||||
Marakhouski et al. [89] | Mesalamine pellets | 1.5 g–3.0 g | 115 | 8 w | Clinical remission: clinical activity index ≤4 | At 3 w: 54/114 p (47 %) | 36/114 p (32 %) | AE: 1/114 p (0.9 %) | 114 p in intention to treat and safety analysis |
Daily dose 1.5 g | |||||||||
Includes p with dose escalation to 3 g/day in nonresponders to initial dose of 1.5 g/day (n = 44) and pts treated with daily dose of 1.5 g (n = 70) | |||||||||
At 8 w: 76/114 p (67 %) | |||||||||
Mesalamine tables | 1.5 g–3.0 g | 118 | At 3 w: 48/115 p (42 %) | 42/118 p (36 %) | 4/118 p (3.4 %) (AE) | 115 p in intention to treat analysis | |||
At 8 w: 78/115 p (68 %) | |||||||||
118 pts in safety analysis | |||||||||
Daily dose 1.5 g | |||||||||
Includes p with dose escalation to 3 g/day in nonresponders to initial dose of 1.5 g/day (n = 52) and p treated with daily dose of 1.5 g (n = 63) | |||||||||
Gibson et al. [90] | Eudragit-L-coated mesalamine | 3 g | 131 | 8 w | Clinical remission: clinical activity index ≤4 | 69 % | 74/131 p (57 %) | 16/131 p (12 %) | |
Ethyl cellulose-coated mesalamine | 3 g | 127 | 69 % | 66/127 p (52 %) | 14/127 p (11 %) | ||||
Ito et al. [43] | pH-dependent release mesalamine | 2.4 g | 66 | 8 w | Decrease in UC activity index | Mean decrease: 1.5 (95 % CI 0.7, 2.3) | 56/66 p (84.8 %) | Not reported | – |
pH-dependent release mesalamine | 3.6 g | 64 | Mean decrease: 2.9 (95 % CI 2.3, 3.5) | 53/64 p (82.8 %) | Not reported | ||||
Time-dependent release mesalamine | 2.25 g | 63 | 1.3 (95 % CI 0.6, 2.1) , | 55/65 p (84.6 %) | Not reported | ||||
p = 0.003 | |||||||||
Difference: 0.2 (95 % CI–0.8, 1.2) |
Oral Mesalamine Preparations Versus Placebo
Several randomized controlled studies and meta-analyses have demonstrated superiority of oral mesalamine preparations over placebo in the induction of remission. In one of the recent meta-analyses published by Ford et al. (included 11 RCTs and 2,086 patients), the relative risk of failure to induce remission with mesalamine compared to placebo was 0.79 (95 % CI 0.73–0.85, p = 0.009) [18]. This meta-analysis also found that the number needed to treat for mesalamine derivatives was 6 (95 % CI 5–8) [18]. Ford et al. did not find any significant differences between the type of oral mesalamine drug and their efficacy in inducing remission in active UC (Cochrane Q = 1.11, p = 0.77) [18].
The Cochrane meta-analysis published in 2006 also found that mesalamine preparations were superior to placebo for induction of remission as well as clinical and endoscopic improvement in patients with active UC. In terms of ability to induce clinical or global improvement or remission, the pooled Peto odds ratio between 5-ASA and placebo was 0.40 (95 % CI 0.30–0.53) [19].
Asacol Versus Placebo
Delayed-release mesalamine preparation like Asacol has been shown to be superior to placebo in two RCTs. Schroeder et al. compared 4.8 and 1.6 g/day of Asacol to placebo. Asacol 4.8 g/day produced statistically significant remission compared to placebo (p < 0.0001), while 1.6 g/day did not induce significant remission rates compared to placebo (p = 0.51) [20]. In another study comparing Asacol to placebo, Snisky found that Asacol 2.4 g/day induced remission in 32 % patients compared to only 9 % by placebo (p = 0.003) [21]
Pentasa Versus Placebo
In a large multicenter double-blind RCT, Pentasa 2 g/day (57 %) and 4 g/day (59 %) produced significantly better clinical improvement compared to placebo, while Pentasa 1 g/day (36 %) could not induce statistically significant remission compared to placebo [22].
MMX Mesalamine Versus Placebo
Lichtenstein et al. demonstrated that MMX mesalamine is superior to placebo in the induction of both clinical and endoscopic remission. In a large multicenter, double-blind RCT, 280 patients were randomized to either MMX mesalamine 4.8 g/day, MMX mesalamine 2.4 g/day, or placebo. MMX mesalamine 4.8 g/day induced remission in 29.2 % of patients, MMX mesalamine 2.4 g/day in 34.2 % patients, and placebo in 12.4 % patients [23].
Olsalazine Versus Placebo
In only RCT comparing clinical remission rates between olsalazine and placebo in patients with active UC, olsalazine was not superior to placebo [18, 24]. But if the results of three trials that compared clinical improvement rates (rather than clinical remission) between olsalazine and placebo were to be included in the analysis, then olsalazine would be superior to placebo (RR of no remission or improvement = 0.81, 95 % CI 0.68–0.96) [18, 25–27].
Balsalazide Versus Placebo
Scherl and colleagues demonstrated that balsalazide (3.3 g twice daily) was better than placebo in achieving remission in patients with mild to moderately active UC. The relative risk of failure to achieve remission between balsalazide and placebo was 0.82 (95 % CI 0.74–0.91) [28].
Oral Mesalamine Preparations Versus Sulfasalazine
The Cochrane meta-analysis updated in 2006 suggested that the newer mesalamine drugs tended toward therapeutic benefit when compared to sulfasalazine in the induction of remission in patients with active UC [19]. The newer mesalamine preparations in comparison to sulfasalazine had a pooled Peto odds ratio of 0.83 (95 % CI 0.60–1.13) for failure to induce global/clinical improvement or remission and 0.66 (95 % CI 0.42–1.04) for failure to induce endoscopic improvement [19]. Also they noticed that newer mesalamine preparations had fewer side effects compared to sulfasalazine [19].
In another meta-analysis by Nikfar and his colleagues found that none of the new mesalamine formulations were superior to sulfasalazine in inducing overall improvement in patients with active UC (overall improvement defined as a positive change in at least two of the following criteria: sigmoidoscopic appearances, histologic appearances, clinical severity, and percentage of bloody stools) [29]. In four trials (three trials comparing delayed mesalamine and one trial comparing Pentasa to sulfasalazine), the relative risk of overall improvement between sulfasalazine and mesalamine was a nonsignificant value of 1.04 (95 % CI 0.89–1.21). In three trials comparing sulfasalazine and olsalazine, the relative risk for overall improvement was 1.14 (95 % CI 0.91–1.43, p = 0.16), again a nonsignificant value [29]. In two trials, comparing sulfasalazine and balsalazide, the relative risk of overall improvement was a nonsignificant value of 1.3 (95 % CI 0.93–1.81, p = 0.12) [29].
Comparison Between Different Mesalamine Drugs for Induction of Remission
There have been few studies and meta-analyses comparing various oral mesalamine preparations in their ability to induce remission in patients with active UC. A meta-analysis by Rahimi et al. found that balsalazide was superior to mesalamine in the induction of both symptomatic and complete remission [30]. In the pooled analysis of three trials comparing balsalazide to mesalamine in their ability to induce symptomatic remission, the relative risk was 1.23 (95 % CI 1.03–1.47, p = 0.0204), while the relative risk for ability to induce complete remission was 1.3 (95 % CI 1.002–1.68, p = 0.0481) [30]. In a meta-analysis by Ford et al., there was no statistically significant difference between the type of mesalamine formulation and their ability to induce remission in patients with active UC (Cochrane Q = 1.11, p = 0.77) [18].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





