CHAPTER 18 Constipation
DEFINITION AND PRESENTING SYMPTOMS
The definition of constipation varies among people, and it is important to ask patients what they mean when they say “I am constipated.” Most persons are describing a perception of difficulty with bowel movements or a discomfort related to bowel movements. The most common terms used by young healthy adults to define constipation are straining (52%), hard stools (44%), and the inability to have a bowel movement (34%).1
The definition of constipation also varies among physicians and other health care providers. The traditional medical definition of constipation, based on the 95% lower confidence limit for healthy adults in North America and the United Kingdom,2 has been three or fewer bowel movements/week. Reports of stool frequency, however, are often inaccurate and do not correlate with complaints of constipation.3 In an attempt to standardize the definition of constipation, a consensus definition was initially developed by international experts in 1992 (Rome I criteria)4 and was revised in 1999 and in 2006 (Rome II and III criteria, respectively; Table 18-1).5,6
Table 18-1 Rome III Criteria for Functional Constipation
| Two or more of the following six must be present*: Manual maneuvers to facilitate at least 25% of defecations (e.g., digital evacuation, support of the pelvic floor) |
* Criteria fulfilled for the previous three months with symptom onset at least six months prior to diagnosis. In addition, loose stools should rarely be present without the use of laxatives, abdominal pain is not required, and there should be insufficient criteria for irritable bowel syndrome. These criteria may not apply when the patient is taking laxatives.
The Rome criteria incorporate the multiple symptoms of constipation, of which stool frequency is only one, and require that a minimum of two symptoms be present at least 25% of the time. Unlike the Rome I criteria, the Rome II criteria include symptoms suggestive of outlet obstruction (e.g., a sensation of anorectal blockage or obstruction and use of maneuvers to facilitate defecation). The Rome III criteria allow patients to have occasional loose stools and require that symptoms be present during the previous three months, with an onset at least six months earlier. When abdominal pain or discomfort is the predominant symptom, irritable bowel syndrome (IBS), rather than constipation, should be considered to be the diagnosis (see Chapter 118). Intermittently loose stools unrelated to laxative use also suggest a diagnosis of IBS. Although distinguishing IBS from constipation alone is important, the symptoms and pathophysiology of these entities overlap substantially.
EPIDEMIOLOGY
PREVALENCE
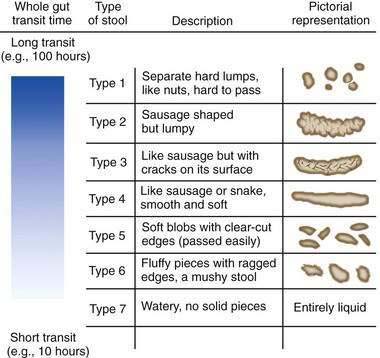
The prevalence of constipation ranges from 2% to 28% of the population in Western countries (Table 18-2)7–17 and varies depending on the demographics of the population, definition of constipation (e.g., self-reported symptoms, fewer than three bowel movements/week, or the Rome criteria), and method of questioning (e.g., postal questionnaire, interview). Some studies have attempted to identify subcategories of constipation based on the symptom pattern. In general, the prevalence is highest when constipation is self-reported9 and lowest when the Rome II criteria for constipation are applied. When the Rome II criteria are used to diagnose constipation, the effects of gender, race, socioeconomic status, and level of education on the prevalence of constipation are reduced.10
INCIDENCE
Little is known about the incidence of constipation in the general population. Talley and colleagues studied 690 nonelderly residents of Olmsted County, Minnesota, at baseline and after 12 to 20 months.18 Constipation, defined as frequent straining at stool and passing hard stool, a stool frequency of fewer than three stools/week, or both, was present in 17% of respondents on the first survey and 15% on the second survey. The rate of new constipation in this study was 50/1000 person-years, whereas the disappearance rate was 31/1000 person-years. Robson and colleagues found that 12.5% of older persons (mean age, 83 years) entering a nursing home had constipation and that constipation developed in 7% over three months of follow-up.19
PUBLIC HEALTH PERSPECTIVE
Constipation results in more than 2.5 million physician visits, 92,000 hospitalizations, and several hundred million dollars of laxative sales/year in the United States.20 Eighty-five percent of physician visits for constipation lead to a prescription for laxatives or cathartics.21 The cost of testing alone in patients with constipation has been estimated to be $6.9 billion annually.22 Among patients with constipation seen in a tertiary referral center, the average cost of a medical evaluation was $2,252, with the greatest cost attributed to colonoscopy.23
In an analysis of physician visits for constipation in the United States between 1958 and 1986, 31% of patients who required medical attention were seen by general and family practitioners, followed by internists (20%), pediatricians (15%), surgeons (9%), and obstetricians-gynecologists (9%). Only 4% of patients were seen by gastroenterologists, suggesting that few such patients were deemed to need advice from a specialist.20,21 In a National Canadian Survey, 34% of persons who reported constipation had seen a physician for their symptoms.9
RISK FACTORS
Risk factors for constipation in the United States include female gender, advanced age, nonwhite ethnicity, low levels of income and education, and low level of physical activity.3,8,11,24 Other risk factors include use of certain medications and particular underlying medical disorders (see later). Diet and lifestyle also may play a role in the development of constipation (Table 18-3).
Table 18-3 Risk Factors for Constipation
GENDER
The prevalence of self-reported constipation is two to three times higher in women than in men,10–1216 and infrequent bowel movements (e.g., once a week) are reported almost exclusively by women.25 In one study of 220 normal subjects eating their normal diets, 17% of women, but only 1% of men, passed less than 50 g of stool daily.26 The reason for the female predominance is unknown. A reduction in levels of steroid hormones has been observed in women with severe idiopathic constipation, although the clinical significance of this finding is dubious.27 An overexpression of progesterone receptors on colonic smooth muscle cells has been reported to down-regulate contractile G proteins and up-regulate inhibitory G proteins.28
In addition, overexpression of progesterone receptor B on colonic muscle cells, thereby making them more sensitive to physiologic concentrations of progesterone, has been proposed as an explanation for severe slow-transit constipation in some women.29
AGE
The prevalence of self-reported constipation among older adults ranges from 15% to 30%, with most,7,21,24,30,31 but not all,8,9,12,17 studies showing an increase in prevalence with age. Constipation is particularly problematic in nursing home residents, among whom constipation is reported in almost half and 50% to 74% use laxatives on a daily basis.32,33 Similarly, hospitalized older patients appear to be at high risk of developing constipation. A study of patients on a geriatrics ward in the United Kingdom showed that up to 42% had a fecal impaction.34
Older adults also tend to seek medical assistance for constipation more commonly than their younger counterparts. In an analysis of physician visits for constipation in the United States between 1958 and 1986, the frequency was about 1% in persons younger than 60, between 1% and 2% in those 60 to 65, and between 3% and 5% in those older than 65 years.21
Constipation in older adults is most commonly the result of excessive straining and hard stools30 rather than a decrease in stool frequency. In a community sample of 209 people ages 65 to 93 years, the main symptom used to describe constipation was the need to strain at defecation; 3% of men and 2% of women reported that their average bowel frequencies were less than three/week.29 Possible causes for the increased frequency of straining in older adults include decreased food intake, reduced mobility, weakening of abdominal and pelvic wall muscles, chronic illness, psychological factors, and medications, particularly pain-relieving drugs.19,32
Constipation is also common in children younger than 4 years.33 For example, in Great Britain, the frequency of a consultation for constipation in general practice was 2% to 3% for children ages 0 to 4, approximately 1% for women ages 15 to 64, 2% to 3% for both genders ages 65 to 74, and 5% to 6% for patients ages 75 years or older. Fecal retention with fecal soiling is a common cause of impaired quality of life and the need for medical attention in childhood.
ETHNICITY
In North America, constipation is reported more commonly by nonwhites than whites. In a survey of 15,014 persons, the frequency of constipation in whites was 12.2%, compared with 17.3% in nonwhites.3 Both groups demonstrate similar age-specific increases in prevalence.7 In developing countries, constipation is less common among the native populations, in whom stool weights are three to four times more than the median of 106 g daily in Britain.26 In rural Africa, constipation appears to be rare.
SOCIOECONOMIC CLASS AND EDUCATION LEVEL
The prevalence of constipation is influenced by socioeconomic status. In population-based surveys, subjects with a lower income status have higher rates of constipation as compared with those who have a higher income.3,6–8 In a survey of approximately 9000 Australians, men and women of lower socioeconomic status were more likely to report constipation than those of higher socioeconomic status.35 Similarly, persons who have less education tend to have an increased prevalence of constipation as compared with those who have more education.3,8,9,11,16
DIET AND PHYSICAL ACTIVITY
Cross-sectional studies have not linked low intake of fiber with constipation,29,36 yet data suggest that increased consumption of fiber decreases colonic transit time and increases stool weight and frequency.22 An analysis from the Nurses Health Study, which assessed the self-reported bowel habits of 62,036 women between the ages of 36 and 61 years, demonstrated that women who were in the highest quintile of fiber intake and who exercised daily were 68% less likely to report constipation than women who were in the lowest quintile of fiber intake and exercised less than once a week.24 Although other observational studies have supported a protective effect of physical activity on constipation, results from trials designed to test this hypothesis are conflicting. In a trial designed to assess the effect of regular exercise on chronic constipation, symptoms did not improve after a four-week exercise program.37 In healthy sedentary subjects, a nine-week program of progressively increasing exercise had no consistent effect on whole-gut transit time or stool weight.38
Dehydration has been identified as a potential risk factor for constipation. Some but not all observational studies have found an association between slowed intestinal transit time and dehydration.36,39 Although patients with constipation are advised routinely to increase their intake of fluid, the benefit of increased fluid intake has not been investigated thoroughly.
MEDICATION USE
Persons who use certain medications are at a substantially higher risk of constipation. In a review of 7251 patients with chronic constipation (and nonconstipated controls) from a general practice database, medications that were significantly associated with constipation were opioids, diuretics, antidepressants, antihistamines, antispasmodics, anticonvulsants, and aluminum antacids (Table 18-4).40 The use of aspirin or other nonsteroidal anti-inflammatory drugs in the older population is associated with a small but significantly increased risk of constipation.14
Table 18-4 Secondary Causes of Constipation
| Mechanical Obstruction |
| Medications |
Anticholinergic agents (e.g., antiparkinsonian drugs, antipsychotics, antispasmodics, tricyclic antidepressants) |
| Metabolic and Endocrinologic Disorders |
| Neurologic and Myopathic Disorders |
COLONIC FUNCTION
LUMINAL CONTENTS
The main contents of the colonic lumen are food residue, water and electrolytes, bacteria, and gas. Unabsorbed food entering the cecum contains carbohydrates that are resistant to digestion and absorption by the small intestine, such as starches and nonstarch polysaccharides (NSPs). Some of the unabsorbed carbohydrate serves as substrate for bacterial proliferation and fermentation, yielding short-chain fatty acids and gas (see Chapter 16). On average, bacteria represent approximately 50% of stool weight.41 In an analysis of feces from nine healthy subjects on a metabolically controlled British diet, bacteria constituted 55% of the total solids, and fiber represented approximately 17% of the stool weight.42
A meta-analysis of the effect of wheat bran on colonic function has suggested that bran increases stool weight and decreases mean colonic transit time in healthy volunteers.42 The effect of bran may be the result primarily of increased bulk within the colonic lumen; the increased bulk stimulates propulsive motor activity. The particulate nature of some fibers also may stimulate the colon. For example, ingestion of coarse bran, 10 g twice daily, was shown to reduce colonic transit time by about one third, whereas ingestion of the same quantity of fine bran led to no significant decrease.41 Similarly, ingestion of inert plastic particles similar in size to coarse bran increased fecal output by almost three times their own weight and decreased colonic transit time.43
ABSORPTION OF WATER AND SODIUM
The colon avidly absorbs sodium and water (see Chapter 99). Increased water absorption can lead to smaller, harder stools. The colon extracts most of the 1000 to 1500 mL of fluid that crosses the ileocecal valve, and leaves only 100 to 200 mL of fecal water daily. Less reabsorption of electrolytes and nutrients takes place in the colon than in the small intestine, and sodium-chloride exchange and short-chain fatty acid transport are the principal mechanisms for stimulating water absorption. Colonic absorptive mechanisms remain intact in patients with constipation. One proposed pathophysiologic mechanism in slow-transit constipation is that the lack of peristaltic movement of contents through the colon allows more time for bacterial degradation of stool solids and increased NaCl and water absorption, thereby decreasing both stool weight and frequency.44 The volume of stool water and quantity of stool solids seem to be reduced proportionally in constipated persons.45
DIAMETER AND LENGTH
A wide or long colon may lead to a slow colonic transit rate (see Chapter 96). Although only a small fraction of patients with constipation have megacolon or megarectum, most patients with dilatation of the colon or rectum report constipation. Colonic width can be measured on barium enema films. A width of more than 6.5 cm at the pelvic brim is abnormal and has been associated with chronic constipation.46
MOTOR FUNCTION
Colonic muscle has four main functions (see also Chapter 98): (1) delays passage of the luminal contents so as to allow time for the absorption of water; (2) mixes the contents and allows contact with the mucosa; (3) allows the colon to store feces between defecations; and (4) propels the contents toward the anus. Muscle activity is affected by sleep and wakefulness, eating, emotion, the contents of the colon, and drugs. Nervous control is partly intrinsic and partly extrinsic by the sympathetic nerves and the parasympathetic sacral outflow.
Transit of contents along the colon takes hours or days (longer than transit in other portions of the gastrointestinal tract). In a study of 73 healthy subjects, the mean colonic transit time was 35 hours.47 In another similar study, the mean colonic transit time in healthy volunteers was 34 hours, with an upper limit of normal of 72 hours.48
Scintigraphic studies in constipated subjects have shown that overall transit of colonic contents is slow. In some patients, the rate of movement of contents is approximately normal in the ascending colon and hepatic flexure but delayed in the transverse and left colon. Other patients show slow transit in the right and left sides of the colon.49
Colonic propulsions are of two basic types, low-amplitude propagated contractions (LAPCs) and high-amplitude propagated contractions (HAPCs).50 The frequency and duration of HAPCs are reduced in some patients with constipation. In one study, 14 chronically constipated patients with proved slow transit of intestinal contents and one or fewer bowel movements weekly were compared with 18 healthy subjects. Four of the patients had no peristaltic movement, whereas peristaltic movement was normal in all the healthy subjects during a 24-hour period. Peristaltic movements in other subjects with constipation were fewer in number and shorter in duration, and thus passed for a shorter distance along the colon, as compared with the findings in the healthy controls. All the healthy subjects reported abdominal discomfort or an urge to defecate during peristaltic movements, and two defecated, whereas only four of the 14 subjects with constipation experienced any sensation during such movements, and none defecated.51
INNERVATION AND THE INTERSTITIAL CELLS OF CAJAL
Proximal colonic motility is under the involuntary control of the enteric nervous system, whereas defecation is voluntary. Slow-transit constipation may be related to autonomic dysfunction.52,53 Histologic studies have shown abnormal numbers of myenteric plexus neurons involved in excitatory or inhibitory control of colonic motility, thereby resulting in decreased amounts of the excitatory transmitter substance P54 and increased amounts of the inhibitory transmitters vasoactive intestinal polypeptide (VIP) or nitric oxide (NO).55
Interstitial cells of Cajal (ICCs) are the intestinal pacemaker cells and play an important role in regulating gastrointestinal motility. They facilitate the conduction of electrical current and mediate neural signaling between enteric nerves and muscles. ICCs initiate slow waves throughout the gastrointestinal tract. Confocal images of ICCs in patients with slow-transit constipation show not only reduced numbers but also abnormal morphology of ICCs, with irregular surface markings and a decreased number of dendrites. In patients with slow-transit constipation, the number of ICCs has been shown to be decreased in the sigmoid colon56 or the entire colon.57,58 Pathologic examination of colectomy specimens of 14 patients with severe intractable constipation has revealed decreased numbers of ICCs and myenteric ganglion cells throughout the colon.59
DEFECATORY FUNCTION
The process of defecation in healthy persons begins with a predefecatory period, during which the frequency and amplitude of propagating sequences (three or more successive pressure waves) are increased. Stimuli such as waking and meals (gastroileal reflex, also referred to as gastrocolic reflex) can stimulate this process. This predefecatory period is blunted, and may be absent, in patients with slow-transit constipation.50 The gastroileal reflex also is diminished in persons with slow-transit constipation. Stool is often present in the rectum before the urge to defecate arises. The urge to defecate is usually experienced when stool comes into contact with receptors in the upper anal canal. When the urge to defecate is resisted, retrograde movement of stool may occur and transit time increases throughout the colon (see Chapter 98).60
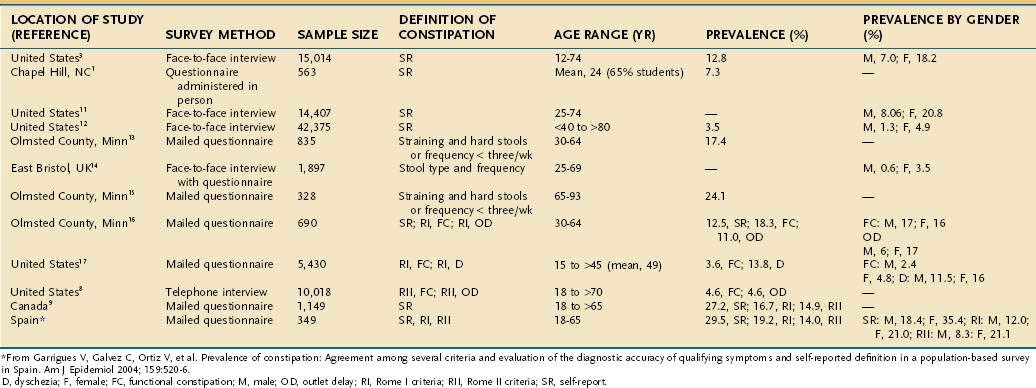
Although the sitting or squatting position seems to facilitate defecation, the benefit of squatting has not been studied in patients with constipation. Full flexion of the hips stretches the anal canal in an anteroposterior direction and straightens the anorectal angle, thereby promoting emptying of the rectum.61 Contraction of the diaphragm and abdominal muscles raises intrapelvic pressure, and the pelvic floor relaxes simultaneously. Striated muscular activity expels rectal contents, with little contribution from colonic or rectal propulsive waves. Coordinated relaxation of the puborectalis muscle, which maintains the anorectal angle, and external anal sphincter at a time when pressure is increasing in the rectum results in expulsion of stool (Fig. 18-1).
The length of the colon emptied during spontaneous defecation varies but most commonly extends from the descending colon to the rectum.62 When the propulsive action of smooth muscle is normal, defecation usually requires minimal voluntary effort. If colonic and rectal waves are infrequent or absent, however, the normal urge to defecate may not occur.51
SIZE AND CONSISTENCY OF STOOL
In a study of normal subjects who were asked to expel single hard spheres of different sizes from the rectal ampulla, the intrarectal pressure and time needed to pass the objects varied inversely with their diameters. Small hard stools are more difficult to pass than large soft stools. When larger stimulated stools were tested, a hard stool took longer to expel than a soft silicone rubber object of approximately the same shape and volume. Similarly, more subjects were able to expel a 50-mL water-filled compressible balloon than a hard 1.8-cm sphere.63
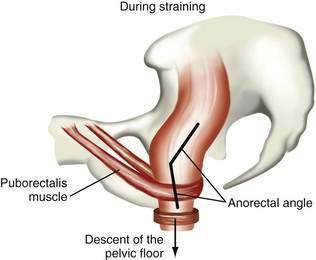
Human stools may vary in consistency from small hard lumps to liquid. The water content of stool determines consistency. Rapid colonic transit of fecal residue leads to diminished water absorption and (perhaps counterintuitively) an increase in the bacterial content of the stool. The Bristol Stool Scale25 is used in the assessment of constipation and is regarded as the best descriptor of stool form and consistency (Fig. 18-2). Stool consistency appears to be a better predictor of whole-gut transit time than of defecation frequency or stool volume.64
CLASSIFICATION
Mechanical small and large bowel obstruction, medications, and systemic illnesses can cause constipation, and these causes of secondary constipation must be excluded, especially in patients presenting with a new onset of constipation (see Table 18-4). Most often, however, constipation is caused by disordered function of the colon or rectum (functional constipation). Functional constipation can be divided into three broad categories—normal-transit constipation, slow-transit constipation, and defecatory or rectal evacuation disorders (Table 18-5). In a study of more than 1000 patients with functional constipation who were evaluated at the Mayo Clinic, 59% were found to have normal-transit constipation, 25% had defecatory disorders, 13% had slow-transit constipation, and 3% had a combination of a defecatory disorder and slow-transit constipation.65
Table 18-5 Clinical Classification of Functional Constipation
| CATEGORY | FEATURES | CHARACTERISTIC FINDINGS |
|---|---|---|
| Normal-transit constipation | Incomplete evacuation; abdominal pain may be present but not a predominant feature | Normal physiologic test results |
| Slow-transit constipation | Infrequent stools (e.g., ≤1/wk); lack of urge to defecate; poor response to fiber and laxatives; generalized symptoms, including malaise and fatigue; more prevalent in young women | Retention in colon of >20% of radiopaque markers five days after ingestion |
| Defecatory disorders (pelvic floor dysfunction, anismus, descending perineum syndrome, rectal prolapse) | Frequent straining; incomplete evacuation; need for manual maneuvers to facilitate defecation | Abnormal balloon expulsion test and/or rectal manometry |
PATHOPHYSIOLOGY
NORMAL-TRANSIT CONSTIPATION
In normal-transit constipation, stool travels along the colon at a normal rate.66 Patients with normal-transit constipation may have misperceptions about their bowel frequencies and often exhibit psychosocial distress.67 Some patients have abnormalities of anorectal sensory and motor function indistinguishable from those in patients with slow-transit constipation.68 Whether increased rectal compliance and reduced rectal sensation are effects of chronic constipation or contribute to the failure of the patients to experience an urge to defecate is unclear. Most patients, however, have normal physiologic testing. IBS with constipation differs from normal-transit constipation in that abdominal pain is the predominant symptom in IBS (see Chapter 118).
SLOW-TRANSIT CONSTIPATION
Slow-transit constipation is most common in young women and is characterized by infrequent bowel movements (less than one bowel movement/week). Associated symptoms include abdominal pain, bloating, and malaise. Symptoms are often intractable, and conservative measures such as fiber supplements and osmotic laxatives are usually ineffective.69,70 The onset of symptoms is gradual and usually occurs around the time of puberty. Slow-transit constipation arises from disordered colonic motor function. Patients who have mild delays in colonic transit have symptoms similar to those seen in persons with IBS.71 In patients with more severe symptoms, the pathophysiology includes delayed emptying of the proximal colon and fewer HAPCs after meals. Colonic inertia is a term used to describe the disorder in patients with symptoms at the severe end of the spectrum. In this condition, colonic motor activity fails to increase after a meal,72 ingestion of bisacodyl,73 or administration of a cholinesterase inhibitor such as neostigmine.74
DEFECATORY DISORDERS
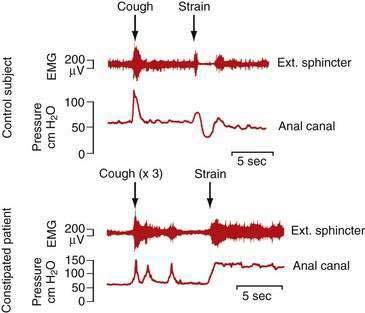
Defecatory disorders arise from failure to empty the rectum effectively because of an inability to coordinate the abdominal, rectoanal, and pelvic floor muscles. Many patients with defecatory disorders also have slow-transit constipation75 Defecatory disorders are also known as anismus, dyssynergia, pelvic floor dyssynergia, spastic pelvic floor syndrome, obstructive defecation, or outlet obstruction. These disorders appear to be acquired and may start in childhood. They may be a learned behavior to avoid some discomfort associated with the passage of large hard stools or pain associated with attempted defecation in the setting of an active anal fissure or inflamed hemorrhoids. Patients with defecatory disorders commonly have inappropriate contraction of the anal sphincter when they bear down (Fig. 18-3). This phenomenon can occur in asymptomatic subjects but is more common among patients who complain of difficult defecation.76 Some patients with a defecatory disorder are unable to raise intrarectal pressure to a level sufficient to expel stool, a disturbance that manifests clinically as failure of the pelvic floor to descend on straining.77
Defecatory disorders are particularly common in older patients with chronic constipation and excessive straining, many of whom do not respond to standard medical treatment.78 Defecatory disorders rarely are associated with structural abnormalities such as rectal intussusception, an obstructing rectocele, megarectum, or excessive perineal descent.79
Patients with defecatory disorders may report infrequent bowel movements, ineffective and excessive straining, and the need for manual disimpaction; however, symptoms, particularly in the case of pelvic floor dysfunction, do not correlate with physiologic findings.80 For a diagnosis of a defecatory disorder, a Rome working group81 has specified the criteria listed in Table 18-6. In patients with this disorder, constipation is functional and caused by dysfunction of the pelvic floor muscles as determined by physiologic tests. Pelvic floor dyssynergia is a subset of these patients in which the anal sphincter fails to relax more than 20% of its basal resting pressure during attempted defecation, despite the presence of adequate propulsive forces in the rectum.
Table 18-6 Rome III Criteria for Functional Defecation Disorders81*
| The patient must satisfy diagnostic criteria for functional constipation (see Table 18-1). |
| During repeated attempts to defecate, the patient must have at least two of the following: Inappropriate contraction of pelvic floor muscles (i.e., anal sphincter or puborectalis) or less than 20% relaxation of basal resting sphincter pressure by manometry, imaging, or EMG |
EMG, electromyography.
* Criteria fulfilled for the previous three months with symptom onset at least six months prior to diagnosis.
Functional fecal retention (FFR) is the most common defecatory disorder in children. It is a learned behavior that results from withholding defecation, often because of fear of a painful bowel movement.82 The symptoms are common and may result in secondary encopresis (fecal incontinence) because of leakage of liquid stool around a fecal impaction. FFR is the most common cause of encopresis in childhood (see Chapter 17).83
DISORDERS OF THE ANORECTUM AND PELVIC FLOOR
RECTOCELE
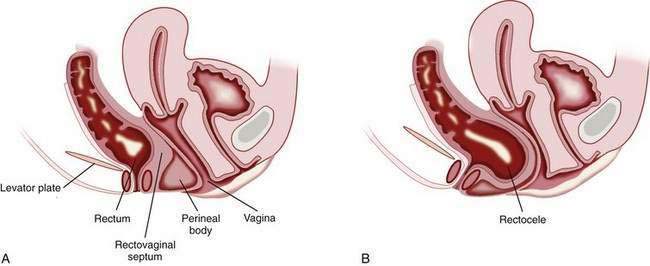
A rectocele is the bulging or displacement of the rectum through a defect in the anterior rectal wall. In women, the perineal body supports the anterior rectal (posterior vaginal) wall above the anorectal junction, and a layer of fascia runs from the rectovaginal pouch of Douglas to the perineal body and adheres to the posterior vaginal wall. The anterior rectal wall is unsupported above the level of the perineal body, and the rectovaginal septum can bulge anteriorly to form a rectocele (Fig. 18-4). Rectoceles can arise from damage to the rectovaginal septum or its supporting structures during vaginal childbirth. These injuries are exacerbated by repetitive increases in intra-abdominal pressure and the long-term effects of gravity. Prolapse of other pelvic organs may be present. For example, urinary incontinence, as well as a previous hysterectomy, has been reported to be more common in patients with a rectocele than in patients with difficult defecation but no demonstrable rectocele.84
Studies using defecating proctography (see later) have shown that rectoceles are common in symptomless healthy women and may protrude as much as 4 cm from the line of the anterior rectal wall without causing bowel symptoms, although 2 cm is the generally accepted lower limit of a rectocele that may be regarded as clinically significant.85 Symptomatic patients report the inability to complete fecal evacuation, perineal pain, sensation of local pressure, and appearance of a bulge at the vaginal opening on straining. Women may report the need to use their thumb or fingers to support the posterior vaginal wall to complete defecation.84 Women also may report the need to use a finger to evacuate the rectum digitally.
Defecating proctography can be used to demonstrate a rectocele, measure its size, and determine whether barium becomes trapped within the rectocele. In one study, trapping of barium in rectoceles changed with the degree of rectal emptying and was related to the size of the rectocele86; however, the size of the rectocele or degree of emptying on defecation has not been shown to correlate with the outcome of surgical repair.87,88
Asymptomatic women with rectoceles do not require surgical treatment. Kegel exercises (designed to strengthen the pelvic floor muscles that support the urethra, bladder, uterus, and rectum) and instructions to avoid repetitive increases in intra-abdominal pressure may help prevent progression of the rectocele. Surgery should be considered only for patients in whom contrast is retained during defecography and patients in whom constipation is relieved with digital vaginal pressure to facilitate defecation.89 Surgical repair can be performed by endorectal, transvaginal, or transperineal approaches. Other types of genital prolapse may also be present, and collaboration between the surgeon and gynecologist may be appropriate. In carefully selected patients surgical repair benefits approximately 75% of patients. In a review of 89 women who underwent a combined transvaginal and transanal rectocele repair for symptoms of obstructive defecation, the repair was successful in 71% of patients, as assessed by the absence of symptoms after one year.90 Reduction in the size of the rectocele, as judged by defecating proctography, does not appear to correlate clearly with improvement in symptoms.88
DESCENDING PERINEUM SYNDROME
In the descending perineum syndrome, the pelvic floor descends to a greater extent than normal (1 to 4 cm) when the patient strains during defecation, and rectal expulsion is difficult. The anorectal angle is widened as a result of pelvic floor weakness, and the rectum is more vertical than normal. The perineal body is weak (thereby facilitating formation of a rectocele), and the lax muscular support favors intrarectal mucosal intussusception or rectal prolapse. The pelvic floor may not provide the resistance necessary for extrusion of solid stool through the anal canal. A common reason for pelvic floor weakness is trauma or stretching during parturition. In some cases, repeated and prolonged defecation appears to be a damaging factor. Symptoms include constipation, incomplete rectal evacuation, excessive straining and, less commonly, digital rectal evacuation.91 Electrophysiologic studies show partial denervation of the striated muscle and evidence of pudendal nerve damage. Histologic examination of operative specimens of the pelvic floor muscles confirms loss of muscle fibers.
DIMINISHED RECTAL SENSATION
The urge to defecate depends in part on tension within the rectal wall (determined by the tone of the circular muscle of the rectal wall), rate and volume of rectal distention, and size of the rectum. Some patients with constipation appear to feel pain normally as the rectum is distended to the maximal tolerable volume, but they fail to experience an urge to defecate with intermediate volumes.92 In a study of women with severe idiopathic constipation, a higher than normal electrical stimulation current applied to the rectal mucosa was required to elicit pain, thereby suggesting a possible rectal sensory neuropathy.93
Rectal hyposensitivity (RH) is defined as insensitivity of the rectum to balloon distention on anorectal physiologic investigation, although the pathophysiology of RH is not entirely clear. Constipation is the most common presenting symptom of RH. In an investigation of 261 patients with RH, 38% had a history of pelvic surgery, 22% had a history of anal surgery, and 13% had a history of spinal trauma.94
RECTAL PROLAPSE AND SOLITARY RECTAL ULCER SYNDROME
Rectal prolapse refers to complete protrusion of the rectum through the anus (see Chapter 125). Occult (asymptomatic) rectal prolapse has been found in 33% of patients with clinically recognized rectoceles and defecatory dysfunction.95 Rectal prolapse can be detected easily on physical examination by asking the patient to strain as if to defecate. A laparoscopic rectopexy—in which the prolapsed rectum is raised and secured with sutures to the adjacent fascia—is the recommended treatment.96
Solitary rectal ulcer syndrome is a rare disorder characterized by erythema or ulceration generally of the anterior rectal wall as a result of chronic straining (see Chapter 115). Mucus and blood may be passed when the patient strains during defecation.97,98 Endoscopic findings may include erythema, hyperemia, mucosal ulceration, and polypoid lesions. Misdiagnosis may occur because of the heterogeneous findings and misleading name of the syndrome (an ulcer need not be present). In a study of 98 patients with solitary rectal ulcer syndrome, 26% were initially diagnosed incorrectly. In patients with a rectal ulcer or mucosal hyperemia, the most common misdiagnoses were Crohn’s disease and ulcerative colitis. In those with a polypoid lesion, the most common misdiagnosis was a neoplastic polyp.99 Histology of full-thickness specimens of the lesion reveals extension of the muscularis mucosa between crypts and disorganization of the muscularis propria. Defecography, transrectal ultrasonography, and anorectal manometry are helpful in the diagnosis.
Varying degrees of rectal prolapse exist in association with solitary rectal ulcer syndrome. Rectal prolapse and paradoxical contraction of the puborectalis muscle can lead to rectal trauma because of the high pressures generated within the rectum. In addition, rectal mucosal blood flow is reduced.100
Medical treatment may be difficult, and a single optimal therapy does not exist. The patient should be advised to resist the urge to strain. Bulk laxatives and dietary fiber may be of some benefit.101 Surgery may be required; rectopexy is performed most commonly. Of patients who undergo surgery for solitary rectal ulcer syndrome with rectal prolapse, 55% to 60% report long-term satisfaction, although a colostomy is eventually required in approximately one third of patients.102 Repair of a rectal prolapse may aggravate constipation. Biofeedback appears to be a promising mode of therapy for patients with solitary rectal ulcer syndrome.103
SYSTEMIC DISORDERS
HYPOTHYROIDISM
Constipation is the most common gastrointestinal complaint in patients with hypothyroidism. The pathologic effects are caused by an alteration of intestinal motor function and possible infiltration of the intestine by myxedematous tissue. The basic electrical rhythm that generates peristaltic waves in the duodenum decreases in hypothyroidism, and small bowel transit time is increased.104 Myxedema megacolon is rare but can result from myxedematous infiltration of the muscle layers of the colon. Symptoms include abdominal pain, flatulence, and constipation.105
DIABETES MELLITUS
The mean colonic transit time is longer in diabetics than in healthy controls. In one study, the mean total colonic transit time in 28 diabetic patients (34.9 ± 29.6 hours; mean ± SD) was significantly longer than that in 28 healthy subjects (20.4 ± 15.6 hours; P < 0.05).106 Among the 28 diabetic patients, 9 of 28 (32%) met the Rome II criteria for constipation and 14 of 28 (50%) had cardiovascular autonomic neuropathy. The mean colonic transit times in diabetic patients with and without cardiovascular autonomic neuropathy were similar. By contrast, a previous study reported that asymptomatic diabetic patients with cardiovascular autonomic neuropathy had significantly longer whole-gut transit times (although still within the range of normal) than a control group without evidence of neuropathy.107 In another study, diabetic patients with mild constipation demonstrated delayed colonic myoelectrical and motor responses after ingestion of a standard meal, whereas diabetics with severe constipation had no increases in these responses after food. Neostigmine increased colonic motor activity in all diabetic patients, suggesting that the defect was neural rather than muscular (see Chapter 35).108
NERVOUS SYSTEM DISEASE
PARKINSON’S DISEASE
Constipation occurs frequently in patients with Parkinson’s disease (PD). In a study of 12 patients with PD compared with normal controls, slow colonic transit, decreased phasic rectal contractions, weak abdominal wall muscle contraction, and paradoxical anal sphincter contraction on defecation were all features in patients with PD and frequent constipation.110 Loss of dopamine-containing neurons in the central nervous system is the underlying defect in PD; a defect in dopaminergic neurons in the enteric nervous system also may be present. Histopathologic studies of the myenteric plexuses of the ascending colon in 11 patients with PD and constipation revealed that in 9 patients, the number of dopamine-positive neurons was one tenth or less the number in control subjects. Dopamine concentrations in the muscularis externa were significantly lower in patients with PD than in controls (P < 0.01).111
Another possible contributor to constipation is the inability of some patients with PD to relax the striated muscles of the pelvic floor on defecation. This finding is a local manifestation of the extrapyramidal motor disorder that affects skeletal muscle. Preliminary observations suggest that injection of botulinum toxin into the puborectalis muscle is a potential therapy for this type of outlet dysfunction constipation in patients with PD.112,113
MULTIPLE SCLEROSIS
Constipation is common among patients with multiple sclerosis (MS). In an unselected group of 280 patients with MS, the frequency of constipation (defined as diminished bowel frequency, digitation to facilitate defecation, or the use of laxatives) was approximately 43%. Almost 25% of the subjects passed fewer than three stools/week, and 18% used a laxative more than once a week. Constipation correlated with the duration of MS but preceded the diagnosis of MS in 45% of subjects. Constipation did not correlate with immobility or the use of medications.114 In another questionnaire study of 221 patients with MS, the frequency of constipation was as high as 54%.115 Constipation in patients with MS can be multifactorial and related to a reduction in postprandial colonic motor activity, limited physical activity, and medications with constipating side effects.
Patients with advanced MS and constipation have evidence of a visceral neuropathy. In a group of patients with advanced MS and severe constipation, all had evidence of disease in the lumbosacral spinal cord and decreased compliance of the colon. Motor and electrophysiologic measurements have shown that the usual increase in colonic motor activity after meals is absent. Among less severely affected patients, slow colonic transit and manometric evidence of pelvic floor muscular and anal sphincter dysfunction have been demonstrated. Patients may have fecal incontinence.116,117 Therapy with biofeedback has been reported to relieve constipation and fecal incontinence, although in a study of 13 patients with MS who underwent biofeedback for either constipation or incontinence, only 38% improved (see Chapter 17).118
SPINAL CORD LESIONS
Lesions Above the Sacral Segments
Spinal cord lesions or injury above the sacral segments lead to an upper motor neuron disorder, with severe constipation. The resulting delay in colonic transit affects the rectosigmoid colon primarily.119,120 In a study of patients with severe thoracic spinal cord injury, colonic compliance was abnormal, with a rapid rise in colonic pressure on instillation of relatively small volumes of fluid. Motor activity after meals did not increase, but the colonic response to neostigmine was normal, thereby suggesting absence of myopathy.
Studies of anorectal function in patients with severe traumatic spinal cord injury have shown that rectal sensation to distention is abolished, although a dull pelvic sensation is experienced by some patients at maximum levels of rectal balloon distention. Anal relaxation on rectal distention is exaggerated and occurs at a lower balloon volume than in normal subjects. Distention of the rectum leads to a linear increase in rectal pressure, without the plateau at intermediate values seen in normal subjects, and ends in high-pressure rectal contractions after a relatively small volume (100 mL) has been instilled into the balloon. As expected, the rectal pressure generated by straining is lower in patients than in control subjects and is less with higher than lower spinal cord lesions. Patients demonstrate a loss of conscious external anal sphincter control, and the sphincter does not relax on straining, suggesting that in normal subjects, descending inhibitory pathways are present.121 These findings explain why some patients with spinal cord lesions experience not only constipation, but also sudden uncontrollable rectal expulsion with incontinence. Other patients cannot empty the rectum in response to laxatives or enemas, possibly because of failure of the external anal sphincter to relax, and they may require manual evacuation.
Electrical stimulation of anterior sacral nerve roots S2, S3, and S4 via electrodes implanted for urinary control in paraplegic patients leads to a rise in pressure within the sigmoid colon and rectum and contraction of the external anal sphincter. Contraction of the rectum and relaxation of the internal anal sphincter persist for a short time after the stimulus ceases. By appropriate adjustment of the stimulus in one study, it was possible for 5 of 12 paraplegic patients to evacuate feces completely and for most of the others to increase the frequency of defecation and reduce the time spent emptying the rectum.122 In another series, left-sided colonic transit time decreased with regular sacral nerve stimulation.123
Lesions of the Sacral Cord, Conus Medullaris, Cauda Equina, and Nervi Erigentes (S2 to S4)
Neural integration of anal sphincter control and rectosigmoid propulsion occurs in the sacral segments of the spinal cord. The motor neurons that supply the striated sphincter muscles are grouped in Onuf’s nucleus at the level of S2. There is evidence that efferent parasympathetic nerves that arise in the sacral segments enter the colon at the region of the rectosigmoid junction and extend distally in the intermuscular plane to reach the level of the internal anal sphincter and proximally to the midcolon via the ascending colonic nerves, which retain the structure of peripheral nerves (see Chapter 98).124
Damage to sacral segments of the spinal cord or to efferent nerves leads to severe constipation. Fluoroscopic studies show a loss of progression of contractions in the left colon. When the colon is filled with fluid, the intraluminal pressure generated is lower than normal, in contrast with the situation after higher lesions of the spinal cord. The distal colon and rectum may dilate, and feces may accumulate in the distal colon. Spasticity of the anal canal can occur. Loss of sensation of the perineal skin may extend to the anal canal, and rectal sensation may be diminished. Rectal wall tone depends on the level of the spinal lesion. In a study of 25 patients with spinal cord injury, rectal tone was significantly higher than normal in patients with acute and chronic supraconal lesions but significantly lower than normal in patients with acute and chronic conal or cauda equina lesions.125
STRUCTURAL DISORDERS OF THE COLON, RECTUM, ANUS, AND PELVIC FLOOR
OBSTRUCTION
Anal atresia in infancy, anal stenosis later in life, or obstruction of the colon may manifest as constipation. Obstruction of the small intestine generally manifests as abdominal pain and distention, but constipation and inability to pass flatus also may be features (see Chapters 96 and 119).
DISORDERS OF SMOOTH MUSCLE
Myopathy Affecting Colonic Muscle
Congenital or acquired myopathy of the colon usually manifests as pseudo-obstruction. The colon is hypotonic and inert (see Chapter 120).
Hereditary Internal Anal Sphincter Myopathy
Hereditary internal anal sphincter myopathy is a rare condition characterized by constipation with difficulty in rectal expulsion and episodes of severe proctalgia fugax, defined as the sudden onset of brief episodes of pain in the anorectal region.126–128 Three affected families have been reported. The mode of inheritance appears to be autosomal dominant with incomplete penetrance. In symptomatic persons, the internal anal sphincter muscle is thickened, and resting anal pressure is increased greatly. In two of the described patients, treatment with a calcium channel blocker improved pain but had no effect on constipation. In another family, two patients were treated by internal anal sphincter strip myectomy; one showed marked improvement and one had improvement in the constipation but only slight improvement in the pain. Examination of the muscle strips showed myopathic changes with polyglucosan bodies (glucose polymers) in the smooth muscle fibers and increased endomysial fibrosis.
Progressive Systemic Sclerosis
Progressive systemic sclerosis (scleroderma) may lead to constipation. In patients with progressive systemic sclerosis and constipation, 9 of 10 had no increase in colonic motor activity after ingestion of a 1000-kcal meal. Histologic examination of colonic specimens from these subjects revealed smooth muscle atrophy of the colonic wall (see Chapter 35).129
Muscular Dystrophies
Muscular dystrophies usually are regarded as disorders of striated muscle, but visceral smooth muscle also may be abnormal. In myotonic muscular dystrophy, a condition in which skeletal muscle fails to relax normally, megacolon may be found, and abnormal function of the anal sphincter is demonstrable.130 Cases associated with intestinal pseudo-obstruction have been reported (see Chapter 120).131
DISORDERS OF ENTERIC NERVES
Congenital Aganglionosis or Hypoganglionosis
Congenital absence or reduction in the number of ganglia in the colon leads to functional colonic obstruction with proximal dilatation, as seen in Hirschsprung’s disease and related conditions (see Chapter 96). In Hirschsprung’s disease, ganglion cells in the distal colon are absent because of an arrest in the caudal migration of neural crest cells in the intestine during embryonic development. Although most patients present during early childhood, often with delayed passage of meconium, some patients with a relatively short segment of involved colon present later in life.132 Typically, the colon narrows at the area that lacks ganglion cells, and the bowel proximal to the narrowing is usually dilated. Two genetic defects have been identified in patients with Hirschsprung’s disease—a mutation in the RET (rearranged during transfection) proto-oncogene, which is involved in the development of neural crest cells, and a mutation in the gene that encodes the endothelin B receptor, which affects intracellular calcium levels.133,134
Hypoganglionosis is reported when small, sparse myenteric ganglia are seen. Neuronal counts can be made on full-thickness tissue specimens and compared with published reference values obtained from autopsy material. Establishing the diagnosis of hypoganglionosis is not easy, because of variations in the normal density of neurons.135 Quantitative declines in the number of neurons in the enteric nervous system also are seen in patients with severe slow-transit constipation and characterized morphologically as oligoneuronal hypoganglionosis.136
Congenital Hyperganglionosis (Intestinal Neuronal Dysplasia)
Congenital hyperganglionosis, or intestinal neuronal dysplasia, is a developmental defect characterized by hyperplasia of the submucosal nerve plexus. Clinical manifestations of the disease are similar to those seen in Hirschsprung’s disease and include young age of onset and symptoms of intestinal obstruction (see Chapter 96). In contrast to functional constipation, affected children do not have symptoms of soiling or evidence of a fecaloma.137
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree