Fig. 2.1
Barium swallow peptic stricture
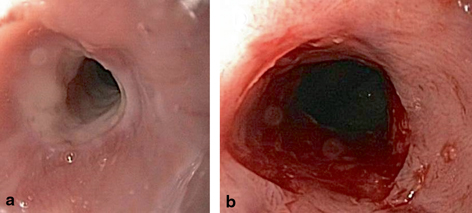
Fig. 2.2
a, b EGD images. Peptic stricture (pre- and post-dilation)
Multiple dilation sessions may be required in order to achieve durable symptom palliation for some patients. A refractory stricture is defined as a fibrotic luminal narrowing resulting in dysphagia for which a luminal diameter of 14 mm cannot be achieved following five consecutive dilation sessions at 2-week intervals, whereas a recurrent stricture is defined as a stricture for which luminal diameter cannot be maintained for 4 weeks following dilation to 14 mm [4]. Adjunctive options for the subset of patients with refractory or recurrent peptic strictures may include temporary placement of an esophageal endoprosthesis (stent) or providing instruction in home dilation techniques [5].
While most gastroenterologists will encounter patients with peptic strictures in the course of general clinical practice, the incidence of peptic stricture is declining overall [6]—likely as a result of the widespread use of prescription and over-the-counter medications which suppress gastric acid production. Following diagnosis of a peptic stricture, proton pump inhibitors (PPI) are typically prescribed to patients not already on chronic antisecretory therapy, in order to reduce the likelihood of stricture recurrence.
Barrett’s Esophagus
Definition
Barrett’s esophagus (BE) was initially described in 1950 by Norman Barrett, a thoracic surgeon, as esophagus with columnar epithelium. Current definitions emphasize both the visible endoscopic presence of salmon-colored mucosa populating the tubular esophagus proximal to the anatomic gastroesophageal junction (Fig. 2.3) and the histopathologic presence of columnar epithelium (Fig. 2.4) as requisite for the diagnosis of BE—this is to be distinguished from columnar epithelium identified in biopsies of an irregular squamocolumnar junction (Z line) or gastric cardia, neither of which constitutes BE. Given these important distinctions, overdiagnosis of BE may be common in clinical practice [7]. Communication and collaboration between the gastrointestinal endoscopist and pathologist may be necessary to ensure that both criteria are met and for confirmation of diagnosis in questionable cases.
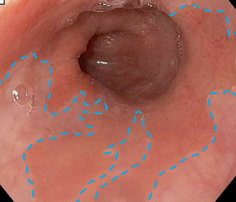
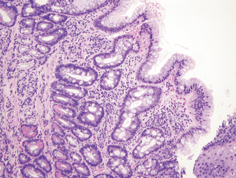

Fig. 2.3
Endoscopic image of BE. The blue line demarcates the borders of the salmon-colored Barrett’s epithelium in the distal esophagus, compared with the lighter pink epithelium of normal squamous mucosa

Fig. 2.4
Barrett’s esophagus, characterized by the presence of columnar epithelium with goblet cells, with maturing surface epithelium (original magnification, 100 ×). (Image courtesy of Chanjuan Shi, MD)
Classic histopathologic findings requisite for the diagnosis of BE have included the presence of columnar epithelium with intestinal metaplasia, as characterized by the presence of goblet cells on Alcian blue stain. This is currently a matter of some controversy. The ability to detect goblet cells may be in part a function of adequate biopsy sampling, with one study suggesting that a minimum of eight forceps biopsies are needed to limit random sampling error and enable optimal detection [8]. Current American Gastroenterological Association (AGA) guidelines require the presence of intestinal metaplasia in the diagnosis of BE [9]. On the other hand, recently updated British Society of Gastroenterology (BSG) guidelines suggest that columnar epithelium without intestinal metaplasia may qualify for the diagnosis of BE [10], under the presumption that columnar epithelium with or without goblet cells is at risk for neoplastic progression .
A common endoscopic stratification system for BE is based on length of visible BE. Short-segment BE (SSBE) is defined as BE with a maximal length of less than 3 cm above the gastroesophageal junction, whereas long-segment BE (LSBE) is defined as BE with a maximal length of ≥ 3 cm. The distribution of BE may not be uniform in all cases, and a validated (Prague) classification scheme endorses endoscopic description of BE by both its circumferential (C) and maximal total (M) length [11]. The development of LSBE is felt to be reflective of a greater pathologic burden of esophageal acid exposure compared with development of SSBE [12], and there are data to suggest that long-term esophageal adenocarcinoma (EAC) risk is greater for LSBE than SSBE [13] .
Cellular Origin and Pathophysiology
Metaplastic transformation of native squamous esophageal epithelium is thought to be induced by chronic inflammation. Development of BE is therefore an esophageal defense mechanism, as the columnar epithelium of BE is relatively acid resistant when compared with squamous mucosa.
Identifying the cellular origin of BE has been a focus of considerable investigation. One rat model of reflux induced by surgical esophagojejunostomy identified progenitor cells of bone marrow origin as candidates for initiation of esophageal metaplasia [14]. An alternative transgenic mouse model implicated stem cells residing in the gastric cardia, activated by bile acid, as progenitors of BE [15].
Rodent models of BE employing reflux disease induced by esophagojejunostomy, with subsequent profound small-bowel esophageal reflux, may not perfectly mimic GERD in humans with intact foregut anatomy. Nonetheless, it is likely that in addition to gastric reflux, duodenal reflux containing bile acids contributes to the pathogenesis and natural history of esophageal neoplasia. Unique effects of bile acids, including deoxycholic acid, on esophageal epithelium include generation of oxidative stress and DNA damage which may contribute to carcinogenesis [16, 17].
It is difficult to pinpoint precisely when BE develops in the course of chronic GERD. In other words, there are no observational data reporting baseline endoscopy in patients with GERD documenting the absence of BE, followed by longitudinal endoscopic surveillance documenting interval development of BE. The potential for development of BE has long been recognized in pediatric patients [18]. BE in pediatric patients has been reported in patients with neurodevelopmental conditions including mental retardation [19] and congenital tracheoesophageal abnormalities. The prevalence of BE in a general pediatric population appears to be less than 1 % [20, 21].
Prevalence and Risk Factors
Not all patients with chronic GERD develop BE. As a corollary, not all patients with BE report regular or frequent heartburn symptoms. In a study of individuals invited to undergo upper endoscopy at the time of screening colonoscopy, BE was detected in 8 % of patients with GERD and 6 % of patients without GERD [22]. The finding of a relatively comparable prevalence of BE among patients with and without GERD has been replicated in multiple studies [23].
Careful review of this data, however, reveals a wide range across studies in the overall prevalence of BE among individuals without GERD, from 1 to 25 % [23]. The high-end estimate of 25 % was obtained from a Veteran’s Affairs population [24], and is reflective of the fact that BE disproportionately affects Caucasian males in the sixth decade of life and older. Consequently, current practice guidelines endorse consideration of age, gender, and ethnicity when determining the appropriateness of screening for BE among individuals with GERD [9].
Anatomic factors associated with development of BE include the presence of a hiatal hernia and obesity . The association between obesity and BE appears to be stronger for central adiposity, defined by such variables as waist circumference and waist-to-hip ratio, than elevated body mass index per se [25–28]. The mechanism underlying the pathway from obesity to BE may not be solely a function of the mechanical effects of obesity in exacerbating GERD. An additional role may be played by the hormonal milieu of obesity, as, for instance, elevated levels of insulin and insulin-like growth factors have been associated with BE [29].
While genetic factors responsible for development of BE have yet to be fully elucidated, familial clustering of BE has been described. Individuals with BE may be diagnosed at a younger age than individuals with sporadic BE. Familial BE likely accounts for less than 10 % of all BE cases [30–33].
Risk of development of BE is almost certainly multifactorial, with likely contributions from environmental as well as host factors. While speculation has focused on the role of alcohol consumption and specific types of alcohols (beer vs. wine vs. liquor), a recent population-based analysis found no evidence of an association between alcohol consumption and risk of development of BE [34]. An intriguing theory involves the evolution of hygiene, the practice of Helicobacter pylori eradication, and emergence of BE. An inverse association has been reported between BE and active H. pylori infection or sequelae of prior H. pylori infection such as chronic atrophic gastritis and gastric intestinal metaplasia [35]. This has led to the hypothesis that H. pylori is protective with respect to the esophagus, and the question as to whether indiscriminate eradication of H. pylori is appropriate in all circumstances [36]. More recent data have identified an altered esophageal microbiome in individuals with GERD and BE compared to normal controls [37].
Esophageal Adenocarcinoma
Incidence and Risk Factors
EAC is the fourth most common gastrointestinal tract malignancy, and the incidence of EAC in the USA and Western Europe has risen considerably over the past several decades. Emerging data suggest that the overall rate of increase in EAC incidence appears to be slowing since the late 1990s [38]. Nonetheless, the rise in incidence of EAC coupled with the decline in incidence of esophageal squamous cell carcinoma has rendered EAC the most commonly encountered esophageal tumor in Western gastroenterology practice.
BE is the major risk factor for EAC. However, the overwhelming majority of cases of EAC are diagnosed in individuals without a known prior diagnosis of BE—presumably because the majority of individuals diagnosed with EAC never experienced GERD symptoms sufficient to warrant earlier endoscopic investigation. In the Northern Ireland Barrett’s Oesophagus Registry, prior diagnosis of BE was present in only 7.3 % of patients diagnosed with EAC [39]. Moreover, while EAC-related mortality is increased considerably among individuals with BE compared to individuals without BE, EAC-specific mortality accounts for only a minority of all-cause mortality in patients with BE. In a meta-analysis of more than fifty studies, EAC accounted for 7 % of deaths among patients with BE. Patients with BE were more than twice as likely to die of a non-esophageal malignancy, which accounted for 16 % of deaths. More than 50 % of deaths were due to cardiovascular or pulmonary disease [40]. Data from such studies challenge the practice of current symptom-targeted screening and surveillance strategies, as shall be discussed.
Risk factors for EAC are similar if not identical to risk factors for BE. Caucasian males are disproportionately represented among individuals diagnosed with EAC. Trends in EAC incidence have paralleled an increasing prevalence of obesity . A recent study investigating trends of EAC and obesity in the USA, the Netherlands, and Spain demonstrated, however, that the increase in incidence of EAC in each of these three nations could not be explained solely by the increasing prevalence of obesity [41]. Additional environmental factors which have been proposed as etiologic agents include dietary nitrogen-containing compounds and H. pylori. Epidemiologic analyses suggest that nonsteroidal anti-inflammatory drugs [42, 43] and statins [44] may have a protective effect .
Progression from BE to EAC
A long-standing estimated progression rate from BE to EAC of 0.5 % per year was based on a study designed to assess for publication bias in the cancer risk of BE [45] . More recent epidemiologic investigations have reported considerably lower progression rates from nondysplastic BE to EAC: 0.38 % per year in an Irish registry [46], 0.30 % per year in a Netherlands registry [47], and as low as 0.12 % per year in a Danish registry, after excluding prevalent cases of EAC diagnosed during an initial period of follow-up [48].
Intermediate steps in the progression from intestinal metaplasia (BE) to EAC can result in histopathologic findings of dysplasia. These features may include nuclear crowding and pleomorphism, hyperchromatism, and emergence of a disorganized epithelial architecture. Dysplasia is currently classified according to one of two grades, based on severity of histopathologic findings: low-grade dysplasia (LGD; Fig. 2.5a) or high-grade dysplasia (HGD; Fig. 2.5b). Based on meta-analysis data, the estimated progression rate from HGD to EAC is between 6 and 7 % per year [49]. The finding of HGD has therefore served as a trigger for therapeutic intervention.
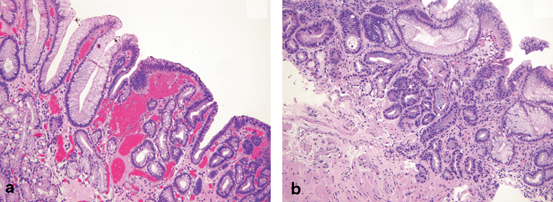

Fig. 2.5
a Barrett’s esophagus with low-grade dysplasia (original magnification, 100 ×). b Barrett’s esophagus with high-grade dysplasia (original magnification, 100 ×). (Images courtesy of Chanjuan Shi, MD)
Conflicting estimates have been reported for progression rates from LGD to EAC. In a multicenter US cohort, the progression rate from LGD to the combined end point of HGD/EAC was less than 2 % per year [50]. This estimate was supported by a recent meta-analysis, in which the annual progression rate from LGD to HGD/EAC (1.7 %) was exceeded by annual mortality due to non-esophageal disease (4.7 %) [51]. On the other hand, studies from the Netherlands have demonstrated that among patients referred with a diagnosis of BE LGD, the majority ( 80 %) are downstaged to less advanced pathology following expert histopathology review [52]; yet among patients with confirmed LGD, progression rates to HGD/EAC exceed 9 % per year [52, 53].
As reflected by the disparate estimates of cancer risk associated with LGD, achieving reliable estimates of risk of progression is contingent upon accurate assessment of baseline prevalent histopathology. Unfortunately, the detection and grading of dysplasia are fraught with numerous challenges. Endoscopic evaluation can fail to detect prevalent dysplasia, as the heterogeneous, nonuniform distribution of dysplasia within a BE segment [54] may elude detection even by systemic biopsy sampling protocols. In addition, the histopathologic grading of dysplasia is by some measure subjective, and interobserver agreement among pathologists is poor [55].
Current Endoscopic Management Approaches to BE and EAC Prevention
A triumvirate approach to management of BE has consisted of endoscopic screening of patients with GERD for diagnosis of BE, endoscopic surveillance of patients with established BE to identify progression to dysplasia and enable early cancer detection, and intervention (historically, surgical esophagectomy) for patients with HGD or early-stage cancer. Numerous factors including revised estimates of BE progression rates, increased recognition of the limitations of symptom-targeted screening and surveillance strategies, and the emergence of endoscopic therapy for BE HGD and stage T1 EAC have all had major impacts on endoscopic management of disease.
Screening
An ideal screening test is an examination with high sensitivity and specificity, an examination which is easy to perform and available at reasonable cost, an examination which is acceptable to patients and clinicians, and an examination which following disease detection offers early treatment of a disease which would otherwise have caused considerable morbidity if diagnosed at a later, symptomatic stage. There are no controlled prospective or retrospective data to suggest that endoscopic screening fulfills all these criteria or prevents or reduces EAC-related mortality.
A rationale for endoscopic screening for BE and EAC has been based on cost-utility analyses, which suggest that a one-time endoscopic screening examination at age 50 or age 60 among individuals with GERD may be cost-effective relative to a no-screening strategy [56, 57]. Such analyses are based on simulated disease models which predict the likelihood of transition between competing health states, and may be sensitive to estimates of BE prevalence, cancer incidence rates, and cost of endoscopy.
One of the major challenges to this screening strategy for EAC prevention is the fact that, as previously discussed, the overwhelming majority of EAC cases are identified in individuals without a known prior diagnosis of BE [39] . Restricting screening to only individuals with symptomatic GERD fails to account for a large asymptomatic population at risk.
Viewed in the context of other accepted cancer screening tests (colonoscopy for colorectal cancer screening, mammogram for breast cancer screening), a one-time screening endoscopy at age 60 may be reasonable among men with GERD—but is difficult to justify in women at any age, given the overall lower age-adjusted incidence rates of EAC among women compared to men [58]. The ambivalence of recent practice guidelines may be viewed as an initial shot across the bow of the current practice of endoscopic screening for BE and EAC. The AGA now recommends against screening for BE among the general population with GERD, albeit with consideration of screening for individuals with risk factors including age 50 years, male gender, white race, and elevated BMI or central adiposity [9]. The BSG states that endoscopic screening for BE is not justified for all individuals with GERD, but can be considered in individuals with chronic symptoms and multiple risk factors [10].
While diagnostic endoscopy has a low overall risk of patient morbidity, the costs of endoscopy are not inconsequential. Both direct costs and indirect costs (i.e., missed time from work) can be significant when considered on a large scale. There may be a future role for disruptive technologies such as unsedated transnasal endoscopy [59] or non-endoscopic methods of tissue acquisition [60] in screening for BE.
Surveillance
The practice of surveillance endoscopy among patients with BE has been similarly justified on the basis of cost-effectiveness analyses. Disease simulation models have demonstrated that a strategy of surveillance at 5-year intervals can be cost-effective compared to a strategy of no surveillance, with the assumption that intervention (esophagectomy) can be offered as an option to those who develop HGD or cancer [61, 62]. These models are sensitive to estimates of cancer risk—and as such, epidemiologic data resulting in lower revised estimates of risk of progression from BE to EAC [46–48] may undermine justification for surveillance.
A recent US-based case-control study reported no evidence of reduced EAC-related mortality among patients with BE who undergo endoscopic surveillance [63]. Alternatively, a recent European study assessed the impact of endoscopic surveillance on all-cause and EAC-specific mortality after stratifying according to endoscopic surveillance intervals. No mortality reduction was identified among individuals receiving “inadequate” surveillance, defined as a time interval 1.5 times expected between initial BE diagnosis and EAC diagnosis accounting for baseline histopathology and grade of dysplasia; there was, however, evidence of 2- and 5-year mortality reduction among those undergoing “adequate” surveillance with endoscopic examination at appropriate frequency [64].
Current AGA practice guidelines recommend surveillance endoscopy at 3–5 year intervals for BE without dysplasia [9]. The BSG guidelines call for modification of the recommended surveillance interval for nondysplastic BE according to length of the BE segment: every 3–5 years for BE length less than 3 cm and every 2–3 years for BE length ≥ 3 cm [10]. The recommended surveillance interval for BE containing LGD is every 6 months [9, 10]. In cases of HGD in which endoscopic therapy is not pursued, the recommended surveillance interval is every 3 months [9].
Given that the majority of patients with BE never develop EAC, a major challenge is to identify individuals with BE who are at risk of development of dysplasia or EAC (progressors) in contrast to those not at risk (nonprogressors). Current risk estimates are based largely on the presence/absence of dysplasia, an imperfect histopathologic marker. Either novel biomarkers or clinical prediction models will be necessary to achieve future optimal risk stratification among individuals with BE.
Endoscopic Therapy
The emergence of endoscopic eradication therapy has had a monumental impact on the management of BE-associated neoplasia. Whereas patients with BE containing HGD or intramucosal EAC once faced surgical esophagectomy as the only treatment option, an increasing proportion of patients are now undergoing endoscopic therapy. In a US cohort from the Surveillance Epidemiology and End Results (SEER) database, for instance, the proportion of patients undergoing endoscopic therapy for HGD or T1 EAC increased from 3 % in 1998 to 29 % in 2009 [65] .
The emergence of endoscopic therapy has been facilitated not only by development of endoscopic techniques for mucosal eradication but also by refined endoscopic staging protocols. Whereas historical cohorts reported high rates of occult invasive cancer among patients undergoing esophagectomy with a preoperative diagnosis of HGD, the rate of occult invasive malignancy has been dramatically reduced with use of endoscopic ultrasound (EUS) for tumor staging [66]. EUS has the ability to both examine the esophageal wall layers for evidence of tumor penetration and assignment of T stage, and also has the ability to identify and sample by fine needle aspiration regional lymph nodes for assignment of N stage.
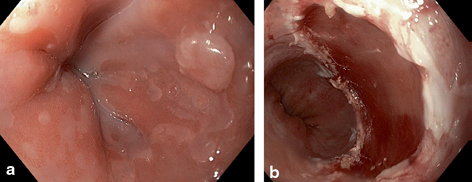
The development of esophageal endoscopic mucosal resection (EMR) techniques has had even more considerable impact in the staging of intramucosal neoplasia. This technique allows the en bloc removal of large segments of the esophageal mucosa and submucosa (Fig. 2.6a, b). This provides a considerable mucosal surface area for histopathologic assessment, overcoming the potential sampling error of limited-size forceps biopsies. In cases of T1 EAC, the resected specimen also enables critical assessment of depth of invasion. The likelihood of lymph node involvement is low (less than 2 %) for T1a (intramucosal) EAC [66]. In many instances, therefore, patients with T1a EAC may undergo endoscopic therapy with reasonable expectation of complete cancer eradication and durable disease remission. The likelihood of lymph node involvement increases considerably, however, for patients with T1b (submucosal invasive) EAC [67] .
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






