Cancer is fundamentally a genetic disease caused by mutational or epigenetic alterations in DNA. There has been a remarkable expansion of the molecular understanding of colonic carcinogenesis in the last 30 years and that understanding is changing many aspects of colorectal cancer care. It is becoming increasingly clear that there are genetic subsets of colorectal cancer that have different risk factors, prognosis, and response to treatment. This article provides a general update on colorectal cancer and highlights the ways that genetics is changing clinical care.
Key points
- •
Impressive declines in incidence and mortality of CRC in the United States have occurred in the last three decades; however, large disparities still exist among African Americans, Hispanics, uninsured, and low-income patients.
- •
The CIN, MIN, and CIMP pathways are the three main known molecular pathways to colorectal cancer, with each containing different histology, risk factors, prognosis, and response to therapy.
- •
Further understanding of genetic makeup of CRC has changed the approach to screening and treatment, with targeted therapy options down the pipeline.
- •
Colonoscopy is the most used CRC screening method in the United States and attention to quality metrics for performance of high-quality colonoscopy is paramount for endoscopists to deliver optimal care.
- •
Care for patients with CRC extends beyond treatment of the initial tumor. Follow-up care of CRC survivors includes surveillance, counseling regarding posttreatment concerns, and counseling to family members of survivors about their increased cancer risk.
Introduction
Cancer is fundamentally a genetic disease caused by mutational or epigenetic alterations in DNA. There has been a remarkable expansion of the molecular understanding of colonic carcinogenesis in the last 30 years and that understanding is changing many aspects of colorectal cancer (CRC) care. This article provides a general update on CRC and highlights how genetics is changing clinical care.
Introduction
Cancer is fundamentally a genetic disease caused by mutational or epigenetic alterations in DNA. There has been a remarkable expansion of the molecular understanding of colonic carcinogenesis in the last 30 years and that understanding is changing many aspects of colorectal cancer (CRC) care. This article provides a general update on CRC and highlights how genetics is changing clinical care.
Molecular pathways
The molecular understanding of colonic carcinogenesis continues to rapidly evolve ( Fig. 1 ). Both hereditary and sporadic CRCs are genetically driven diseases. Hereditary CRC syndromes are caused by germline mutations and sporadic CRC is driven by alterations in DNA structure (mutations) or function (epigenetics).
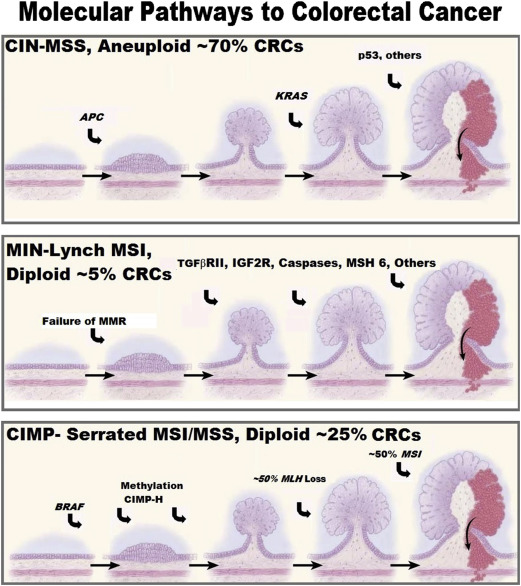
About 6% of all CRCs are caused by a hereditary syndrome for which the genetic basis has been identified. Germline mutations in more than 15 genes that are required for DNA repair and/or regulation of signaling pathways that affect DNA repair are now known to cause an increased risk of CRC. These syndromes can be nonpolyposis syndromes, such as Lynch syndrome, or can be colonic polyposis syndromes with development of scores to thousands of adenomatous, hamartomatous, and/or serrated polyps ( Table 1 ).
| Syndrome | Genes | Functions |
|---|---|---|
| Lynch syndrome | MLH-1 , MSH-2 , MSH-6 , PMS-2 EPCAM | Required for DNA mismatch repair |
| Familial adenomatous polyposis | APC | Regulates Wnt signaling |
| MUTYH -associated polyposis | MUTYH | Required for DNA base excision repair |
| Proofreading polymerase-associated polyposis | POLD1 , POLE | Required for proofreading and repair of polymerase errors during DNA replication |
| Hereditary mixed polyposis | GREM1 | Bone morphogenic protein antagonist, regulates transforming growth factor-β signaling |
| Familial juvenile polyposis | SMAD4 , BMPR1 | Regulates transforming growth factor-β signaling |
| Peutz-Jeghers syndrome | STK-11 | Kinase that regulates multiple signaling pathways |
| Cowden disease, Bannayan-Riley-Ruvalcaba syndrome | PTEN | Negative regulator of AKT signaling |
| CHEK2 | CHEK2 | Negative regulator of cyclin-dependent kinases |
The three major pathways to CRC (chromosomal instability [CIN], microsatellite instability (MIN)/Lynch, and CpG island methylator phenotype [CIMP]/serrated) are described in Fig. 1 . Both the CIN and MIN pathways are thought to progress through the classic adenoma-carcinoma sequence, whereas the CIMP pathway is a serrated polyp-carcinoma sequence. As a first approximation, CRCs from these pathways are distinguished based on microsatellite stability (MSS) and the mutational status of KRAS and BRAF as shown in Fig. 1 but this is an oversimplification: the mutational/epigenetic composition of CRCs is highly varied with the average CRC containing around 90 different mutations. The Cancer Genome Atlas separated CRCs into hypermutated (16% of CRCs; median mutation number, 728) and nonhypermutated (median of 58 mutations) groups and found that the mutational profiles of the groups differ. Several more complex classification systems are being developed in hopes of more precisely predicting biologic behavior and response to therapy. It is becoming increasingly clear that there are genetic subsets of CRCs that have different risk factors, prognosis, and response to treatment. This article highlights how the genetic understanding of CRC is changing almost all facets of CRC care.
Public health burden
The global incidence of CRC was estimated to be about 1.4 million in 2012; it is the third most common cancer worldwide accounting for about 10% of the total cancer burden. There is up to a 10-fold difference in CRC incidence and mortality around the world, with higher rates generally found in high-income countries. Time trends show incidence and mortality are continuing to increase in low-income countries but have stabilized or are declining in many high-income countries.
There has been an impressive decline in the United States over the last 30 years in the incidence (36%) and mortality (50%) of CRC ( Fig. 2 ) that has been attributed largely to CRC screening. Nonetheless, CRC remains the fourth most common cancer (>134,000 cases) and second leading cause of cancer-related mortality (>49,000 deaths) in the United States with substantial demographic disparities in CRC outcomes.
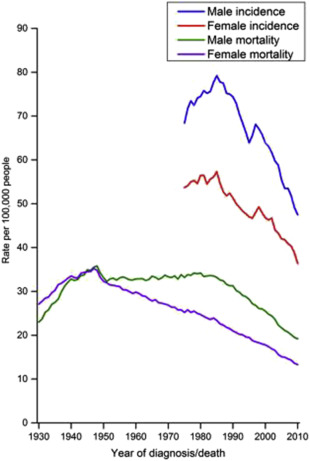
CRC rates are decreasing in all age groups except those younger than age 50 where an increase in rectal cancer has been seen. Reports from Surveillance, Epidemiology, and End Results CRC registry data initially identified concerning increased incidence of young adult CRCs with an annual percentage increase of about 1.5% to 2% for patients 20 to 49 years of age.
African Americans have the poorest CRC outcomes in the United States, are diagnosed at a later stage of disease, and have about a 10% lower 5-year survival than white persons. These disparities are thought to be largely caused by differences in access to care. In 2012, 70% of white persons compared with 66% of African Americans aged 50 and older ever had a screening colonoscopy or sigmoidoscopy. Similarly, screening rates are lower among Hispanics (56.8%) and persons with annual incomes less than $15,000 (53.8%). For uninsured individuals, screening rates are particularly low, with one study citing 33% of uninsured patients undergoing any test for CRC screening compared with 77% of insured patients. The identification of geographic “hotspots” of high CRC mortality within the United States by Siegel and colleagues may be caused by poverty and racially driven decreased access to care.
Impact of Genetics on Colorectal Cancer Burden
Genetic factors play an important role in CRC risk and predisposition. Not only is CRC driven by genetic and epigenetic alterations in DNA (see Fig. 1 ), about 25% of patients with CRC have a family history of the disease (familial CRC) and about 6% of CRCs are caused by a defined hereditary syndrome. Currently germline mutations in more than 15 different genes (see Table 1 ) have been shown to cause hereditary CRC. It is now recognized that there is substantial clinical overlap between CRC hereditary syndromes, such as Lynch syndrome, and hereditary breast and ovarian cancer syndrome and that the phenotype of several polyposis syndromes overlap substantially. This, along with the dramatically decreasing costs of next-generation sequencing, has led to a marked increase in cancer gene panel use to assess high-risk families, which in turn has improved the understanding of the phenotypic expression of these genetic syndromes. Genome-wide association studies have identified at least 23 single-nucleotide polymorphisms that are associated with modest increases in CRC risk. Lastly, it is likely that gene-environment interactions are responsible for at least some of the risk in the remaining families with multiple CRCs.
Risk and protective factors
Increasing age, male sex, African American race, and birth/residence in high-income countries are well established nonmodifiable risk factors for CRC. Although observational studies have consistently reported obesity, lack of physical activity, alcohol/tobacco use, and diets high in red/processed meats and low in fruits/vegetables/fiber are associated with an increased CRC risk, it is not clear to what extent changing these risk factors in an adult modulates risk. Nonetheless, prevention recommendations for CRC ( Box 1 ) are similar to those for cardiovascular disease prevention and seem prudent.
Get screened
Avoid tobacco
Limit alcohol consumption
Stay lean
Get regular physical activity
Limit red meat intake
Avoid processed meat
Increase intake of fiber-containing foods including fruits and vegetables
Consider aspirin if you are also at high risk for cardiovascular disease
Impact of Genetics
Genetic factors not only influence the likelihood of developing many of these risk factors (obesity, alcohol and tobacco use), but undoubtedly also modulate the risk of CRC development in individuals with these risk factors. Among a large number of potential chemopreventive approaches, the strongest body of evidence supports aspirin use and other nonsteroidal anti-inflammatory drugs in this setting. A large number of observational and interventional studies have shown long-term aspirin use is associated with substantial (20%–40%) decreases in sporadic adenoma incidence, metachronous adenoma incidence, sporadic CRC incidence, and mortality and a decreased risk of metastases in CRCs. The beneficial effects of aspirin on colonic carcinogenesis seem to be modulated by genetic susceptibility. Nan and colleagues showed the protective effect of aspirin differed dramatically as a function of two single-nucleotide polymorphisms at chromosomes 12 and 15. Observational studies have also suggested that aspirin may improve overall survival in patients with CRC, with the effect being more pronounced in PIK3CA mutated tumors. Liao and colleagues reported a dramatic survival effect of aspirin use in patients with PIK3CA mutant with an HR for CRC-specific survival of 0.18 (0.06–0.61) and overall survival of 0.54 (95% confidence interval, 0.31–0.94), with no effect in patients with PIK3CA wild-type CRCs (hazard ratio, 0.96; 95% confidence interval, 0.69–1.32; and hazard ratio, 0.94; 95% confidence interval, 0.75–1.17, respectively). A recent systematic review and meta-analysis found heterogeneity in the results but concluded the benefit of postdiagnosis aspirin treatment on overall mortality in CRC is more pronounced in PIK3CA mutated tumors.
Screening and early detection
CRC screening is the standard of care in the United States. The US Preventive Services Task Force and all the gastrointestinal and cancer societies recommend colon cancer screening in the general population starting by age 50 (some recommend by age 45 in African Americans) ( Table 2 ). Screening for CRC can prevent CRC by identification and removal of colonic polyps in addition to early detection of CRCs. The concept of precancerous polyps eventually giving rise to CRC was suggested as early as 1927. The recognition by Dukes in 1932 that CRC survival was better in earlier stage disease birthed the notion that removal of precancerous polyps and detection of early stage CRC could reduce the impact of CRC. The history of CRC screening has been recently chronicled, and indeed much of the recent decline in CRC incidence and mortality in the United States (see Fig. 2 ) is thought to be caused by these efforts.
| Colon Cancer Prevention Screening | 2008 USMSTF Followup Recommendations | 2015 USPSTF Followup Recommendations |
|---|---|---|
| Flexible Sigmoidoscopy | 5 y (+/− FOBT or FIT every y) | 5 y or every 10 y + Annual FIT |
| Colonoscopy | 10 y | 10 y |
| Double Contrast Balloon Enema | 5 y | Not recommended |
| CT Colonography | 5 y | 5 y |
| Colon Cancer Detection Screening | ||
| High Sensitivity Guiac Fecal Occult Blood Test or High Sensitivity Fecal Immunochemistry Test | Annual | Annual |
| Stool DNA Test | Interval Uncertain | 1 or 3 y a |
CRC screening is discussed in detail elsewhere in this issue (see Anderson BW, Ahlquist, DA: Molecular Detection of Gastrointestinal Neoplasia: Innovations in Early Detection and Screening , in this issue) but the current status of screening tests is summarized here. CRC screening tests recommended in the United States are divided into those that primarily detect cancer (fecal occult blood tests [FOBT], fecal immunochemical tests [FIT], stool DNA tests) and those that can also detect polyps (flexible sigmoidoscopy [FS], colonoscopy, computed tomography colonography). Multiple controlled trials have shown that guaiac-based FOBTs decrease CRC mortality by 12% to 33%. Although FITs have not been studied in controlled trials with cancer mortality as the end point, they are preferred over guaiac-based FOBTs based on their higher sensitivity for CRC. Stool DNA tests, in turn, have a higher sensitivity but lower specificity than FITs for CRC and advanced adenomas.
FS has been shown in controlled trials to reduce CRC mortality by about 25%. Despite a lack of controlled trials, case-control and cohort studies have indicated superiority of colonoscopy over FS, making colonoscopy the preferred intervention because of its higher sensitivity for CRC and adenoma detection compared with FS. Several controlled trials are currently underway comparing the effectiveness of colonoscopy to FOBTs including one in the United States (the CONFIRM trial in the United States funded by the Veterans Administration [ ClinicalTrials.gov NCT01239082 ]); the final results of these trials are still a decade away.
Although arguments can be made for which CRC screening test is superior, the best screening test is the one that gets done and done well. Determinants of which screening options are feasible in a country depends largely on health resource availability. The World Gastroenterology Organization has outlined a resource-based cascade of CRC screening options to provide a framework for health care systems to do the best with the available resources and the Organization is actively engaged in many countries to promote affordable screening options and provide training in endoscopic skills in low-resource countries.
Although there are quality issues relevant to all CRC screening tests, colonoscopy is thought to be the most operator-dependent. Thus, substantial efforts have been made to improve colonoscopy quality. Missed adenomas are thought to be the most common reason for interval CRCs (CRCs that occur before the next recommended screening examination). The adenoma detection rate (ADR), which is the fraction of patients undergoing screening colonoscopy who had one or more adenomas detected, inversely correlates with interval CRC risk and has been adopted by the American College of Gastroenterology and American Society for Gastrointestinal Endoscopy as perhaps the most important quality indicator in screening colonoscopy. The initial targets for ADR (25% for men; 15% for women) were set at levels slightly below the mean detection rates of adenomas in screening colonoscopy studies. ADRs have been increasing progressively with improvements in colonoscopic equipment, preparation quality, and endoscopic technique; recent studies report many endoscopists have ADRs exceeding 40% or even 50%. Based on these data, recommended ADR targets were recently revised to 30% for men and 20% for women.
Endoscopists with ADRs less than 25% should take steps to improve their ADR. One predictor of a low ADR is short withdrawal time (WT; time of mucosal inspection during withdrawal of the scope from the cecum). It is recommended that WTs be recorded as a colonoscopy quality measure and that average WT should be 6 minutes or more in screening colonoscopies. Endoscopists with low ADRs and short WTs should slow down and take more time to inspect the colonic mucosa and improve their technique (examining proximal portion of folds, suctioning pools of liquid, adequate distention of the colonic lumen). Although incomplete resection of polyps is also thought to be an important contributor to interval cancers, quality measures have not yet address this issue.
Impact of Genetics on Colorectal Cancer Screening
Genetic factors have long been used to stratify risk, modify screening recommendations, and recently genetic screening for sporadic CRCs has become available. Screening recommendations for genetically defined hereditary CRC syndromes typically involve much more intense screening regimens often with annual colonoscopy starting in adolescence or young adulthood. Similarly, individuals with one first-degree relative with CRC younger than age 60 or greater than one first-degree relative with CRC but no identifiable hereditary syndrome are advised to use colonoscopy every 5 years as the preferred screening strategy and to start screening at age 40 or 5 to 10 years younger than the earliest CRC in the family. A recent study reported that patients with a family history of CRC had modestly higher overall screening rates than those without, but screening rates in the 40 to 49 age group in high-risk families was less than 40%, leaving substantial room for improvement.
The first DNA-based test was recently approved by the Food and Drug Administration and Centers for Medicare/Medicaid Services for CRC screening in the general population based on a large controlled trial showing that a multitarget stool test that includes FIT and a panel of genetic and methylated DNA markers (Cologuard) was superior to FIT alone for detecting CRCs (sensitivity, 92% vs 74%) and advanced adenomas (sensitivity, 42% vs 24%). A blood test for methylated septin 9 is clinically available for CRC screening in Europe and parts of Asia with a reported 85% sensitivity for CRC but a low sensitivity for advanced adenomas (≈20%). Additional blood-based genetic screening tests are currently in development.
Screening protocols and effectiveness of CRC screening vary as a function of the molecular basis of the CRC. The adenoma-carcinoma sequence progresses more rapidly in the microsatellite instability (MSI)/Lynch pathway so colonoscopy screening is recommended more frequently (1–2 years regardless if polyps are found). CRC screening also seems to be less effective for MSI CRCs (arising from either the MIN/Lynch or the CIMP/serrated pathway; see Fig. 1 ); MSI is two to three times more common in interval CRCs than in noninterval CRCs, although it is not clear whether this is caused by more rapid progression of this pathway or that serrated polyps are more difficult to identify and remove compared with conventional adenomas.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






