Colonic Adenomas and Carcinomas
Colorectal carcinoma is one of the most common neoplasms affecting individuals living in industrialized nations. Colorectal cancer is the fourth ranking cancer worldwide, accounting for approximately 9% of all cancers (1). The lifetime risk of developing colorectal carcinoma in the United States is approximately 5.5% for both men and women, with an approximately 2% chance of dying from the disease (2). Most carcinomas develop from adenomas, their precursor lesion. These adenomas occur sporadically or as part of a polyposis syndrome (see Chapter 12). Adenomas are benign by definition, although they are neoplastic and may harbor an invasive carcinoma. Carcinomas also arise in areas of dysplasia in patients with idiopathic inflammatory bowel disease (IBD) (see Chapter 11).
Considerable advances have occurred in our understanding of the molecular events associated with the progression of colorectal carcinoma since the publication of the first edition of this book. Complex interactions between inherited and acquired genomic and other biologic changes associate with both benign and malignant large bowel neoplasia. Colon cancer is highly curable if it is diagnosed in its early stages.
Incidence
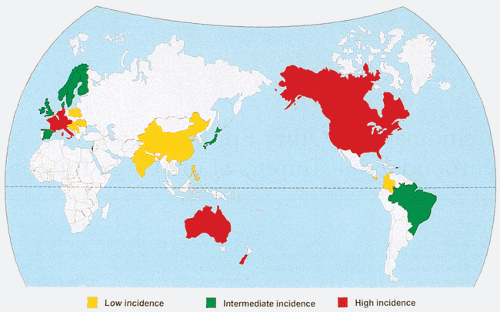
Colorectal cancer exhibits at least a 25-fold variation in occurrence worldwide (1,3). It is most common in the industrialized countries of Western and Eastern Europe, North America, New Zealand, and Australia (Fig. 14.1), while its incidence is low in Africa and Asia (1,4). These large geographic differences in colorectal cancer incidence are most likely explained by different environmental and dietary exposures. Migrants from countries where colon cancer risk is low to high-risk countries show a rapid increase in their colon cancer rates (1,5). A rapid increase in colorectal cancer frequency occurring in Japan parallels its increasing prosperity and westernization (4). This phenomenon is equivalent to a migration in time rather than space and suggests that indigenous Japanese, like those who have migrated to the United States, will acquire levels of colorectal cancer risk equivalent to, or higher than, those of U.S. whites. In fact, colorectal cancer rates for Japanese individuals born in the United States are now higher than those for U.S. whites (6).
The overall and subsite frequency of colorectal cancer also shows considerable intranational ethnic variation, a phenomenon best documented in the United States and New Zealand. It is difficult to determine whether these differences have a genetic or environmental basis, given the different cultural and socioeconomic backgrounds of the ethnic groups within these countries. In the United States, the highest incidence rates in the Surveillance, Epidemiology, and End Results (SEER) registries for the years 2000 to 2003 were in Black males (72.9 of 100,000) followed by White males (61.4 of 100,000), Black females (56.1 of 100,00), Asian males (51.2 of 100,000), Hispanic males (47.3 of 100,000), White females (44.7 of 100,000), Asian females (35.7 of 100,000), and Hispanic females (32.7 of 100,000) (2).
Mortality
In 2006, an estimated 55,170 people died of colorectal cancer in the United States (2). Overall, the age-adjusted mortality rate for colorectal cancer among all ethnic groups in the United States has declined since 1975 (2). However, SEER mortality data for the years 2000 to 2003 show a significantly higher mortality for black Americans with colorectal cancer than their white compatriots (2). Mortality rates are lowest for Asian and Hispanic colorectal cancer patients in the United States.
Etiology
It is important to recognize that colon cancers and rectal cancers associate with different risk factors. This is consistent with the observation that colon cancers rise in
frequency among migrants from low- to high-risk areas, while rectal cancers exhibit a fairly stable incidence (4). Moreover, the colonic subsites also show considerable variation in cancer incidence and in cancer-associated risk factors. Thus, right-sided cancers generally constitute a larger proportion of colorectal cancers in low-risk populations than in high-risk groups and show smaller postmigration increases than do left-sided cancers. That said, it should be noted that the progression of the neoplastic process from adenoma to carcinoma is similar throughout all segments of the large bowel, and that the risk factors for carcinoma and adenoma are similar (7).
frequency among migrants from low- to high-risk areas, while rectal cancers exhibit a fairly stable incidence (4). Moreover, the colonic subsites also show considerable variation in cancer incidence and in cancer-associated risk factors. Thus, right-sided cancers generally constitute a larger proportion of colorectal cancers in low-risk populations than in high-risk groups and show smaller postmigration increases than do left-sided cancers. That said, it should be noted that the progression of the neoplastic process from adenoma to carcinoma is similar throughout all segments of the large bowel, and that the risk factors for carcinoma and adenoma are similar (7).
Environmental and genetic influences probably play roles at different points in the neoplastic progression. Several well-characterized colon cancer syndromes indicate that inherited susceptibility plays an important role in the pathogenesis of colorectal cancer (see Chapter 12). Conversely, epidemiologic, experimental, and migrant studies clearly indicate environmental influences in the genesis of the disease. Diet may alter endogenous characteristics, such as the bowel flora, which in turn influence the conversion of ingested foods into potential carcinogens. Other environmental factors, such as physical activity, occupational exposures, or ethanol, may further influence these interactions. The interaction of environmental factors with genetic factors is complex and currently under intense scrutiny.
Genetic Factors
Familial forms of colon cancer fall into several categories: (a) patients with a polyposis syndrome, (b) patients with defined colon cancer family syndromes, and (c) patients who appear to have sporadic cancers but who have other family members with colon cancer. Genetic influences are best defined in two autosomal dominant syndromes: Familial adenomatous polyposis (FAP) and hereditary nonpolyposis colon cancer syndrome (HNPCC) (see Chapter 12). Hereditary polyposes account for approximately 1% of all colorectal carcinomas, HNPCC accounts for another approximately 5%, and perhaps 30% or more of sporadic carcinomas may be inherited (8).
Neoplasia in Asymptomatic First-degree Relatives of Persons with Colon Cancer
The presence of a colorectal cancer in a first-degree relative (sibling or parent) represents an important colon cancer risk factor. It may increase a person’s lifetime risk from 1.8-fold to as high as eightfold that of the general population (9). The effect of family history is greatest for younger individuals (i.e., those younger than 45 years of age). Asymptomatic patients with one first-degree relative with colorectal cancer have nearly double the risk of developing adenomas or
cancers than asymptomatic individuals without a family history; the cancer often affects younger persons (9,10,11,12). The incidence of colonic neoplasms among first-degree relatives of colorectal cancer patients ranges from 15% to 20% (13). These trends are even more pronounced in those having more than one affected relative (14,15). Familial clusters of colorectal cancer could arise on the basis of a shared gene pool, a shared environment, or a combination of both.
cancers than asymptomatic individuals without a family history; the cancer often affects younger persons (9,10,11,12). The incidence of colonic neoplasms among first-degree relatives of colorectal cancer patients ranges from 15% to 20% (13). These trends are even more pronounced in those having more than one affected relative (14,15). Familial clusters of colorectal cancer could arise on the basis of a shared gene pool, a shared environment, or a combination of both.
Relationship between Colon Cancer and Energy Balance
Reduced physical activity and obesity have emerged as consistent epidemiologic associations with colon cancer risk (7,16,17,18,19,20,21,22,23) and probably account for the association of this cancer with sedentary occupations, weight gain since age 25, body mass index, family income, and the rural/urban gradient in colon cancer frequency. Colon cancer is also associated with central adiposity independent of body mass index (22). Excess energy intake over energy expenditure may also account, at least in part, for the association of colon cancer with fat intake, since fat is a major source of energy (16). It is of interest that rectal cancer does not share these risks (7). The mechanism that accounts for the association between a positive energy balance and colon cancer has not been identified, but it has been proposed that physical activity may stimulate hormonal release, activating neural reflex mechanisms that enhance propagative peristalsis and increased colonic motility. This, in turn, leads to decreased mucosal contact with intraluminal carcinogens.
In addition, the insulin resistance that commonly affects obese individuals may also play a role (24). Type 2 diabetes associates with a threefold increase in colorectal cancer risk compared with the nondiabetic population (25). In addition, colon cancer patients exhibit greater degrees of glucose intolerance and insulin resistance than patients without colorectal cancer (26). Insulin has growth and metabolic effects that could impact the colonic epithelium predisposing to cancer development. Insulin stimulates proliferation and inhibits apoptosis in colorectal cancer cell lines, and promotes growth of colon tumors in experimental animals (27,28,29). The metabolic effects of insulin lead to increased concentrations of glucose and triglycerides that may additionally result in an environment in which transformed colonocytes have greater available energy sources. Insulin may also affect cell-signaling pathways by activating insulinlike growth factor, protein kinase C, and mitogen-activated protein (MAP) kinase, leading to increased mitotic activity and potential carcinogenesis (30).
Nongenetic Host Factors
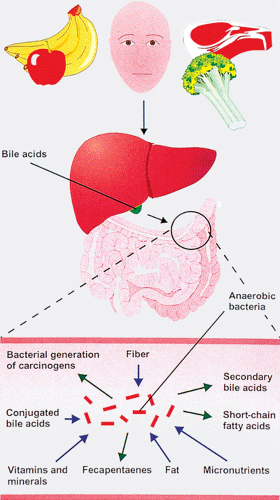
Intestinal epithelium is exposed to a complex luminal environment that plays an etiologic role in colorectal tumorigenesis. Variably digested food passes into the colonic lumen from the small bowel and mixes with bile acids and other luminal constituents (Fig. 14.2). The luminal contents vary with the diet. Millions of bacteria are present in the gut lumen, and many of these generate energy by degrading and fermenting plant cell material (31). The effects of the bacterial flora are noted in Table 14.1. One byproduct, butyrate, slows cell proliferation and facilitates access of DNA repair enzymes.
Dietary Factors
The vast literature associated with the relation of diet to colorectal cancer continues to generate controversy. This is
due, in part, to differences in the methods employed to measure dietary variables and to the comparatively small increases in risk that can be demonstrated with even the strongest dietary associations. The three methods for dietary studies are ecologic studies that relate cancer incidence to per capita food consumption, and two analytic methods—the case control method, which assesses dietary practices of patients who have been diagnosed with cancer, and prospective cohort studies, which measure dietary exposures in healthy subjects who are followed until cancer develops. Each method has built-in flaws. Ecologic studies do not identify the dietary patterns of specific cancer patients, which may actually differ from those of the general population. Dietary data collected prospectively in cohort studies may not hold true for the future, and dietary measurements taken at the time of cancer diagnosis may reflect the influence of disease upon food preferences rather than past experience. Moreover, complex interrelationships exist between energy balance, fat and meat consumption, fiber, alcohol intake, and micronutrients, making it difficult to tease out the relative impact of any one variable upon the risk of colon cancer. The following discussion summarizes the pertinent trends in this field of study.
due, in part, to differences in the methods employed to measure dietary variables and to the comparatively small increases in risk that can be demonstrated with even the strongest dietary associations. The three methods for dietary studies are ecologic studies that relate cancer incidence to per capita food consumption, and two analytic methods—the case control method, which assesses dietary practices of patients who have been diagnosed with cancer, and prospective cohort studies, which measure dietary exposures in healthy subjects who are followed until cancer develops. Each method has built-in flaws. Ecologic studies do not identify the dietary patterns of specific cancer patients, which may actually differ from those of the general population. Dietary data collected prospectively in cohort studies may not hold true for the future, and dietary measurements taken at the time of cancer diagnosis may reflect the influence of disease upon food preferences rather than past experience. Moreover, complex interrelationships exist between energy balance, fat and meat consumption, fiber, alcohol intake, and micronutrients, making it difficult to tease out the relative impact of any one variable upon the risk of colon cancer. The following discussion summarizes the pertinent trends in this field of study.
TABLE 14.1 Effects of Colonic Bacterial Flora | |
|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree