Chapter 3.11
Coeliac disease and nutrition
Imran Aziz and David S. Sanders
Royal Hallamshire Hospital, Sheffield, UK
Coeliac disease is a common condition affecting up to 1% of the adult population. Delays in diagnosis are common. The average time delay for patients with symptoms prior to the diagnosis being made is 13 years. For every adult case detected, it is estimated that there are eight cases not detected. Patients with coeliac disease have an associated morbidity and mortality. In addition, quality of life studies suggest that the majority of patients benefit from a gluten-free diet (GFD). Furthermore, the GFD reduces or alleviates the risk of associated complications. This chapter will discuss how our conceptual understanding of coeliac disease has evolved over the years and also the impact of a GFD.
3.11.1 Diet and modernisation
Although humankind may have existed in some progressive form for 2.5 million years, it is only in the last 10,000 years that we have been exposed to wheat. Wheat was originally cultivated in the Fertile Crescent (south western Asia) with a farming expansion that lasted from ~9000 BC to 4000 BC. Thus it could be considered that wheat and therefore gluten is a relatively novel introduction to the human diet [1]. Gluten is a high molecular weight heterogeneous compound which occurs in the endosperm of wheat but also in rye and barley, that can be fractionated to produce alpha, beta and gamma peptides.
Prior to 1939 and the outbreak of World War II, the rationing system had already been devised. This led to an imperative to try and increase agricultural production. Thus it was agreed in 1941 that there was a need to establish a Nutrition Society. A meeting of workers interested in nutritional problems was convened by Sir John Orr and held at the Royal Institution [2]. The main objective of the new society was to provide a common meeting place for workers in various fields of nutrition. The very roots of the society were geared towards necessarily increasing the production of wheat [2]. This goal was achieved and by the end of the 20th century, global wheat output had expanded five-fold.
3.11.2 Aetiopathogenesis and prevalence
Coeliac disease, a chronic inflammatory disorder of the small intestine, can be defined as a state of heightened immunological responsiveness to ingested gluten (from wheat, barley or rye) in genetically susceptible individuals [3]. Historically, coeliac disease was felt to be a rare condition with an estimated prevalence of 1 in 8000 [4]. In addition, most clinicians expected to recognise infant or childhood presentations with overt symptoms of malabsorption, in the form of diarrhoea and weight loss (or faltering growth). However, there has been a paradigm shift in our conceptual understanding of coeliac disease. With the advent of endoscopic small intestinal biopsies and new serological assays, the prevalence of this condition is now widely appreciated to be around 1% [5]. Adult presentations are now more frequent than paediatric with a ratio of 9:1 with patients most commonly presenting between the ages of 40 and 60 years [7].
3.11.3 Clinical features
It is now recognised that patients do not always have to present with classic GI symptoms of malabsorption, with low Body Mass Index (BMI) accounting for only 5% of all cases diagnosed, with most having normal or overweight BMI [8]. Far more commonly, patients describe non-classic symptoms [9], including atypical GI symptoms consistent with irritable bowel syndrome (such as bloating, abdominal discomfort, gas or altered defaecation [10]), or present insidiously with iron deficiency anaemia [11], osteoporosis [12], ataxia or peripheral neuropathy [13]. Patients who present in this way may be initially overlooked because of the lack of GI symptoms. Finally, some individuals may have the potential to develop coeliac disease (Figure 3.11.1).
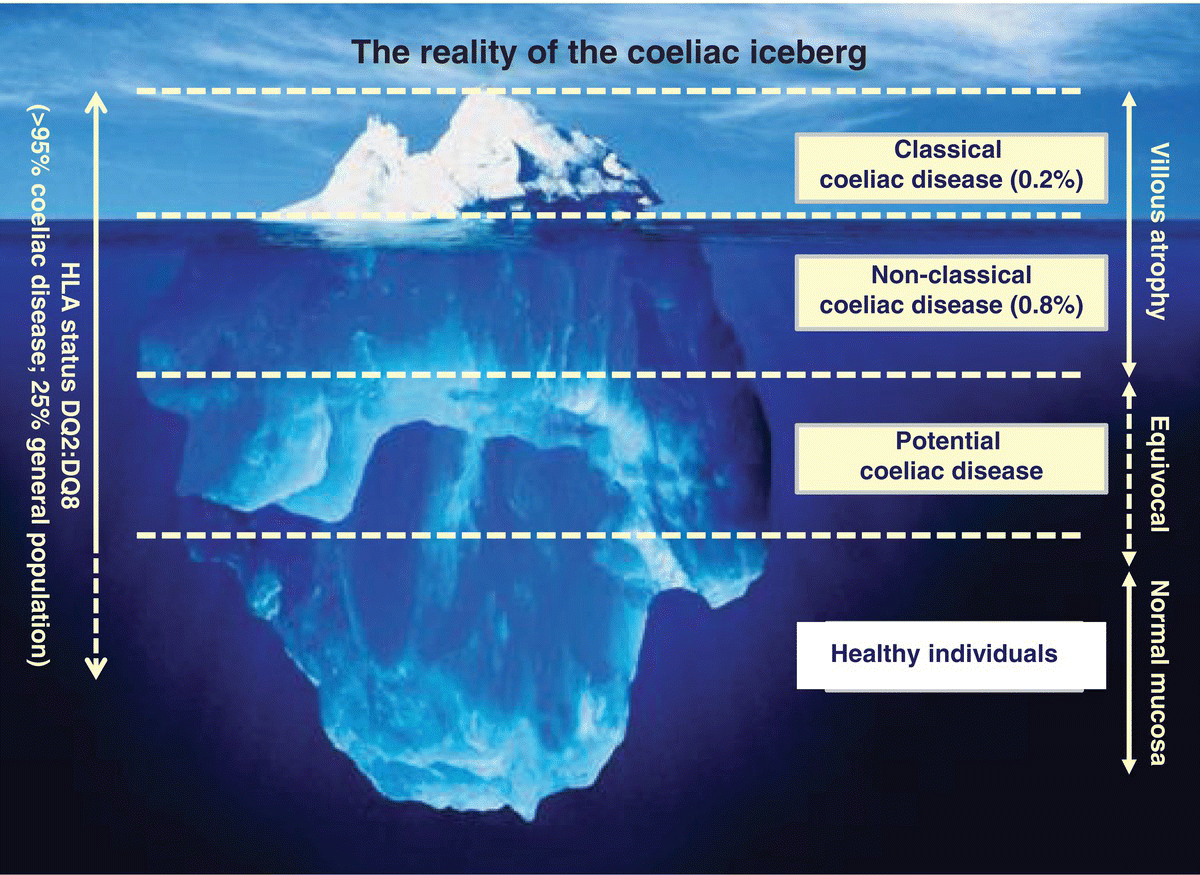
Figure 3.11.1 The coeliac iceberg, model showing the hidden forms of coeliac disease that lie below the waterline. HLA, human leucocyte antigen.
3.11.4 Diagnosing coeliac disease
The gold standard diagnosis of coeliac disease requires duodenal biopsies showing villous atrophy (flattened small intestine) [3,14]. There are a number of serological tests that have been reported to be accurate in identifying patients who should then be referred for a duodenal biopsy. Serological testing for coeliac disease has evolved over the years, initially starting with antigliadin antibodies (AGA) followed by endomysial (EMA) and tissue transglutaminase (tTG) antibodies. Due to their poor diagnostic accuracy, as evidenced by their lower sensitivity and specificity, AGA have largely been superseded by EMA and tTG for routine serological testing in coeliac disease. More recently, there has been interest in deamidated gliadin antibodies and point of care tests [15,16].
The human leucocyte antigens (HLA) DQ2 and/or DQ8 are closely linked with coeliac disease, occurring in up to 98% of cases, but are also present in 25% of the normal population [17,18]. This suggests that other unidentifiable factors, in addition to the correct HLA typing, play a role in the development of coeliac disease. Recent genetics and genome-wide association studies have identified non-HLA loci that may be contributory to the development of coeliac disease [19].
Testing for the HLA DQ2/DQ8 susceptibility genes is not recommended in routine clinical practice as it is an expensive test, not readily available and thus should be reserved for equivocal cases where it can be used as a negatively predictive test [20]. If an individual does not have HLA DQ2 or 8 then it is very unlikely that they have coeliac disease.
There is now growing evidence to suggest that a group of patients may complain of gluten-related symptoms despite the absence of diagnostic markers for coeliac disease, such as negative coeliac serology and normal duodenal biopsies. This is a newly recognised clinical entity, termed non-coeliac gluten sensitivity (NCGS) [21,22]. Currently, we do not fully understand the natural history or indeed the pathophysiology of NCGS. Nevertheless, these individuals do clinically benefit from a GFD.
3.11.5 Dietary causes
There has been recent interest in the effects of feeding practices during infancy on the risk of coeliac disease developing in genetically susceptible individuals. Breast milk seems to have a protective effect although it is not clear whether this is merely delaying or preventing coeliac disease [23]. In order to shed further light on the relationship between breastfeeding, gluten introduction and the prevention of coeliac disease, research is currently under way in 10 European centres studying the influence of infant nutrition, and that of genetic, immunological and environmental factors, on the risk of developing coeliac disease [24]. Current ESPGHAN Committee on Nutrition recommendations and the findings of a recent systematic review suggest that gluten should be introduced into the infant’s diet between months 4 to 7 and whilst the infant is being breastfed [25,26].
3.11.6 Dietary and non-dietary effects
Although some patients with coeliac disease may present with iron deficiency anaemia, the non-specific symptom of ‘tiredness all the time’ is very common [27,28]; this is attributed to the presence of a low ferritin, folate or vitamin B12. These nutritional deficiencies may occur in up to 50% of coeliac patients at the time of presentation. Generally, these deficiencies correct on a GFD.
Recent population-based studies have described only a modestly increased risk of malignancy and mortality in patients with coeliac disease [29]. Importantly, this risk appears to fall as time from diagnosis increases (in those patients who adhere to a GFD) [30]. Although small intestinal lymphoma may be 50 times more common in an individual with coeliac disease, the annual incidence is low (0.5–1 per million), so the absolute risk for patients with coeliac disease is modest.
Coeliac disease is known to cause metabolic bone disease, with 32–80% of adult coeliac patients having bone mineral density (BMD) measurements more than one standard deviation below the population mean [31]. Corazza et al. demonstrated that those with non-classic disease do not have loss of BMD or metabolic bone derangement to the extent of those with classic disease [32]. Other small studies support this finding [33].
The importance of reduced BMD lies in its translation to fracture risk. In a large population-based cohort study comprising 4732 subjects with coeliac disease, West et al. found a very modest overall increased risk of fracture (hazard ratio 1.3) [34].
Infertility, subfertility and an increased risk of an adverse outcome during pregnancy (miscarriage, low birth weight and intrauterine growth retardation) have all been attributed to undiagnosed coeliac disease. However, these risks may be less than historically described.
Functional hyposplenism has been shown to occur in 30% of patients with coeliac disease. For this reason, Haemophilus influenzae, pneumococcal and annual influenza vaccination should be offered to patients with evidence of hyposplenism on blood film [6,35].
Patients with coeliac disease have a reduced quality of life (QOL) and increased likelihood of anxiety and depression in comparison to age- and sex-matched healthy controls, or even those with other organic diseases such as inflammatory bowel disease [36]. Furthermore, it has recently been established that there is an increased prevalence of irritable bowel syndrome and reflux disease in patients with coeliac disease, compared to controls, which accounts for further reductions in QOL and mental status. QOL may therefore be improved if patients with coeliac disease were also assessed and managed for reflux and irritable bowel syndrome [36].
In an obesogenic environment, it has been suggested that undetected coeliac disease may confer a benefit to individuals. It has recently been shown that the mean total cholesterol was 4.84 mmol/L in newly diagnosed adults with coeliac disease (n = 100). Men had 21% lower and women had 9% lower mean total cholesterol in comparison with the general population. There was no change in mean total cholesterol following a GFD. However, there was a small but statistically significant increase of 0.12 mmol/L in the mean HDL cholesterol. Thus there appears to be little benefit conferred to patients with undetected coeliac disease [37].
Recent work pertains to the role of detecting coeliac disease in adult patients with type 1 diabetes. The prevalence of coeliac disease amongst children and adults with type 1 diabetes in the UK has been shown to be 3.3–4.4% [38,39]. At diagnosis, adult type 1 diabetes patients with undetected coeliac disease have worse glycaemic control (8.2% versus 7.5%), lower total cholesterol (4.1 versus 4.9), lower HDL cholesterol (1.1 versus 1.6), and a higher prevalence of retinopathy (58.3% versus 25%), nephropathy (45% versus 5%) and peripheral neuropathy (42.9% versus 15%). After 1 year on a GFD, only the lipid profile improved overall, but in adherent individuals HbA1c and markers for nephropathy also improved. Furthermore, treament with a GFD in this study was safe and there was no difference in QOL after 1 year on a GFD [39]; this suggests that the institution of a GFD in patients with an already complex diabetic diet does not adversely affect QOL [39].
3.11.7 Dietary treatment
The cornerstone of treatment for coeliac disease is lifelong adherence to a strict GFD, which can be a major and initially overwhelming undertaking. For the majority of patients, a GFD leads to clinical and histological remission, normalisation of standardised mortality rate, a reduction in long-term health complications (i.e. osteoporosis) and in some studies, an improvement in psychological well-being and QOL [40,41]. QOL improves after 1 year on a GFD and may be sustained in the long term. Patients with coeliac disease on a GFD have a reduced QOL compared to healthy controls but this is still an improvement from their undiagnosed state.
Patients with potential coeliac disease, as defined by positive coeliac serology but unremarkable duodenal biopsies (see Figure 3.11.1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






