Celiac disease is common, affecting approximately 1 in 100 people, yet it remains underdiagnosed. This article reviews our current understanding of celiac disease, diagnosis, and common pitfalls. Although the cornerstone of treatment is a gluten-free diet, some patients may still have persisting symptoms and warrant further investigations.
- •
Celiac disease affects 1% of the population but the diagnosis is often missed or delayed.
- •
Serology is inadequate for diagnosis; a small-bowel biopsy should demonstrate villous atrophy.
- •
Up to one-third of patients have persisting symptoms, usually because of inadvertent gluten ingestion.
- •
Conditions associated with chronic diarrhea in celiac disease include microscopic colitis, pancreatic insufficiency, small bowel bacterial overgrowth, and lactose intolerance.
- •
Refractory celiac disease is rare. Selected cases may respond to immunosuppression.
Contemporary celiac disease
Celiac disease is defined as a state of heightened immunologic responsiveness to ingested gluten in genetically susceptible individuals. Epidemiologic studies screening cohorts of healthy adult volunteers in the United States, United Kingdom, and other European countries consistently report a prevalence of 0.5% to 1.0%. There is some evidence that the prevalence is increasing. Despite being a common, global phenomenon, diagnosis is often delayed.
Definitions
The first diagnostic criteria for celiac disease by the European Society of Pediatric Gastroenterology and Nutrition in 1969 required a structurally abnormal mucosa while taking gluten, a clear improvement in villous structure while on gluten-free diet (GFD), and relapse of the mucosal changes on gluten challenge. This 3-step approach reflects the thinking of the day that celiac disease was a permanent condition that started in childhood. The subsequent 40 years have seen an evolution in endoscopy, the development of highly accurate serologic tests, and large epidemiologic studies that have contributed to our current understanding of celiac disease. It is now recognized that most patients may have more subtle presenting symptoms, adult presentations are more frequent than pediatric, and there is a “pre-celiac” state.
Recognition of the celiac iceberg has improved understanding and detection of celiac disease ( Fig. 1 ). The visible iceberg above the waterline depicts patients with typical gastrointestinal symptoms, such as diarrhea and weight loss. The subsequent stratum just below the waterline represents patients considered to have atypical presentations. They may have vague, nonspecific gastrointestinal symptoms, such as bloating, or conditions associated with celiac disease, such as iron deficiency anemia, osteoporosis, and persistently abnormal liver function tests. Patients are often identified in screening groups, which would include those with type 1 diabetes, autoimmune thyroid disease, or a first-degree relative with celiac disease.

The deeper layers of the iceberg represent the “pre-celiac” state. Latent celiac disease describes a patient with a normal small bowel mucosa while on a gluten-containing diet who later develops celiac disease. It has been shown that before the development of villous atrophy, some patients may have symptoms, such as abdominal pain, weight loss, and diarrhea, or complications, such as osteoporosis. Furthermore, these may resolve with GFD. Latent celiac disease also describes the converse situation: a patient with biopsy-proven celiac disease who later has a normal small-bowel biopsy despite a normal, gluten-containing diet. One recent study found that 13 of 61 adults with biopsy-proven celiac disease in childhood now following a normal diet had normal mucosa at repeat biopsy. Two of the 13 with latent disease relapsed at subsequent follow-up. In this carefully selected group, up to 18% may have true latency and immune tolerance to gluten.
Recently, the National Institutes of Health consensus group used the term latent celiac disease to describe individuals with positive serology and normal biopsy and this has been adopted by some researchers. The term “potential celiac disease” can also be used to describe positive serology with normal biopsy. This may be found in first-degree relatives of patients with celiac disease, as well as those with autoimmune diseases, such as hypothyroidism. The term gluten sensitivity may be used interchangeably with potential celiac disease and is defined as a condition of some morphologic, immunologic, or functional disorder that responds to gluten exclusion. This includes gluten-sensitive diarrhea and immunologic response to gluten in family members of celiac patients.
Diagnosis
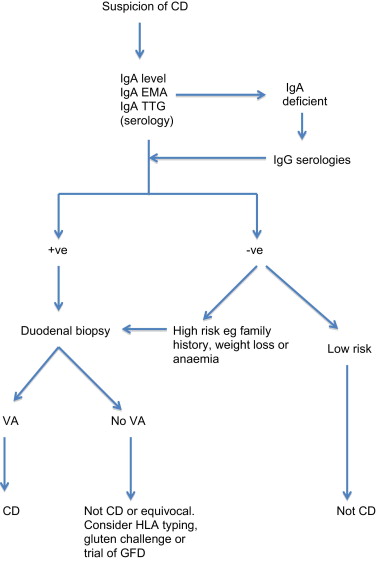
As demonstrated by the celiac iceberg, the presence of relevant symptoms or serologic testing alone is not sufficient to confirm the diagnosis. A simple diagnostic algorithm is shown in Fig. 2 .
Serology
Endomysial antibody (EMA) and tissue transglutaminase (TTG) have a combined sensitivity and specificity of more than 90% when used in combination in selected, high celiac prevalence populations. However, when the prevalence of celiac disease falls to 1%, as found in screening populations, the positive predictive value of these tests falls to approximately 80% or less. The sensitivity of the serologic tests also falls well below 90% when histologic grades less than Marsh 3 (villous atrophy) are considered. This is a clinical problem that is difficult to evaluate, as most studies have excluded patients without villous atrophy. Pooled data for diagnostic accuracy of serologic testing is therefore precluded by the heterogeneous nature of the studies. Instead, ranges for sensitivity and specificity are given in Table 1 . It is also important to note that there is no linear relationship between adherence to GFD, mucosal recovery, and serology. It is possible to have normalization of serology with persisting villous atrophy. Likewise, a clinical response to GFD is not predictive of mucosal recovery. Indeed, although most patients report a subjective improvement on GFD, complete mucosal recovery may occur in only 8% of patients. Serology alone is inadequate for diagnosis. False-positive TTG occurs in conditions such as inflammatory bowel disease, autoimmune disease, and chronic liver disease. Conversely antibody-negative disease accounts for about 9% (4%–22%) of cases. Causes of antibody-negative celiac disease include selective immunoglobulin A (IgA) deficiency, immunosuppressive therapy, and self-imposed gluten-free diet. Of course the possibility that the villous atrophy is not caused by celiac disease must also be considered.
| Test | Sensitivity Range, % | Specificity Range, % |
|---|---|---|
| IgA EMA | 68–100 | 89–100 |
| IgA TTG | 38–100 | 25–100 |
| IgA a-DGP | 79–98 | 80–95 |
A new class of assays detecting the presence of antibodies to deamidated synthetic peptides of gliadin has shown high diagnostic performance equivalent to conventional tests. A recent meta-analysis does not suggest any diagnostic advantage is conferred by the use of these newer assays. Likewise, point-of-care testing (finger-prick testing) has shown early promise but currently lacks the sensitivity and specificity required to replace conventional serology.
HLA
A negative test for HLA DQ2 and DQ8 has a high negative predictive value and is useful to exclude celiac disease. This may have a role in those with borderline histology or serology, unwilling to give up a self-imposed GFD, or with a family history. HLA status may also be used to risk stratify first-degree relatives. Based on a gene dose effect, clinicians in the future may be able to quantify the risk of developing celiac disease to be low or high depending on the presence or absence of homozygosity.
Biopsy
The patchy nature of the histologic change in celiac disease is well documented. For this reason, it is recommended to take multiple small-bowel biopsies to minimize sampling error. Since the advent of fiberoptic endoscopy, biopsies are usually taken from the second part of the duodenum. This has been shown to be equivalent to jejunal biopsy for the detection of villous atrophy. A recent advance in this field is the demonstration that optimal strategies for diagnosing celiac disease can only achieve 100% sensitivity by always including a duodenal bulb biopsy. Historically, bulb biopsy was avoided because of a belief that Brunner glands interfered with interpretation of atrophy. However, this belief is not evidence based. In one prospective study, 9% (n = 126) of adults with newly diagnosed celiac disease had villous atrophy in the bulb only. No control patient had villous atrophy in the bulb alone. Furthermore, causes of villous atrophy other than celiac disease are rare. Non-celiac villous atrophy should be considered in “nonresponsive celiac disease” and causes include peptic inflammation, Giardia , Helicobacter infection, and Crohn disease. Histologic grades that fall short of villous atrophy, ie, Marsh grade 1 and 2, are a gray area. There is some evidence that suggests this situation should be managed as celiac disease with villous atrophy. One group randomized 23 patients with positive EMA, compatible HLA status, and Marsh grade 1 or 2 changes to receive either normal diet or GFD. Those on GFD had improvement symptomatically, serologically, and histologically. Those on normal diet showed persistence or worsening of all parameters. This finding is supported by others. However, caution should be exercised because these lesser changes are not uncommon and are not always attributable to celiac disease. Other causes include drugs, immune dysregulation, and infection.
Diagnosis
As demonstrated by the celiac iceberg, the presence of relevant symptoms or serologic testing alone is not sufficient to confirm the diagnosis. A simple diagnostic algorithm is shown in Fig. 2 .
Serology
Endomysial antibody (EMA) and tissue transglutaminase (TTG) have a combined sensitivity and specificity of more than 90% when used in combination in selected, high celiac prevalence populations. However, when the prevalence of celiac disease falls to 1%, as found in screening populations, the positive predictive value of these tests falls to approximately 80% or less. The sensitivity of the serologic tests also falls well below 90% when histologic grades less than Marsh 3 (villous atrophy) are considered. This is a clinical problem that is difficult to evaluate, as most studies have excluded patients without villous atrophy. Pooled data for diagnostic accuracy of serologic testing is therefore precluded by the heterogeneous nature of the studies. Instead, ranges for sensitivity and specificity are given in Table 1 . It is also important to note that there is no linear relationship between adherence to GFD, mucosal recovery, and serology. It is possible to have normalization of serology with persisting villous atrophy. Likewise, a clinical response to GFD is not predictive of mucosal recovery. Indeed, although most patients report a subjective improvement on GFD, complete mucosal recovery may occur in only 8% of patients. Serology alone is inadequate for diagnosis. False-positive TTG occurs in conditions such as inflammatory bowel disease, autoimmune disease, and chronic liver disease. Conversely antibody-negative disease accounts for about 9% (4%–22%) of cases. Causes of antibody-negative celiac disease include selective immunoglobulin A (IgA) deficiency, immunosuppressive therapy, and self-imposed gluten-free diet. Of course the possibility that the villous atrophy is not caused by celiac disease must also be considered.
| Test | Sensitivity Range, % | Specificity Range, % |
|---|---|---|
| IgA EMA | 68–100 | 89–100 |
| IgA TTG | 38–100 | 25–100 |
| IgA a-DGP | 79–98 | 80–95 |
A new class of assays detecting the presence of antibodies to deamidated synthetic peptides of gliadin has shown high diagnostic performance equivalent to conventional tests. A recent meta-analysis does not suggest any diagnostic advantage is conferred by the use of these newer assays. Likewise, point-of-care testing (finger-prick testing) has shown early promise but currently lacks the sensitivity and specificity required to replace conventional serology.
HLA
A negative test for HLA DQ2 and DQ8 has a high negative predictive value and is useful to exclude celiac disease. This may have a role in those with borderline histology or serology, unwilling to give up a self-imposed GFD, or with a family history. HLA status may also be used to risk stratify first-degree relatives. Based on a gene dose effect, clinicians in the future may be able to quantify the risk of developing celiac disease to be low or high depending on the presence or absence of homozygosity.
Biopsy
The patchy nature of the histologic change in celiac disease is well documented. For this reason, it is recommended to take multiple small-bowel biopsies to minimize sampling error. Since the advent of fiberoptic endoscopy, biopsies are usually taken from the second part of the duodenum. This has been shown to be equivalent to jejunal biopsy for the detection of villous atrophy. A recent advance in this field is the demonstration that optimal strategies for diagnosing celiac disease can only achieve 100% sensitivity by always including a duodenal bulb biopsy. Historically, bulb biopsy was avoided because of a belief that Brunner glands interfered with interpretation of atrophy. However, this belief is not evidence based. In one prospective study, 9% (n = 126) of adults with newly diagnosed celiac disease had villous atrophy in the bulb only. No control patient had villous atrophy in the bulb alone. Furthermore, causes of villous atrophy other than celiac disease are rare. Non-celiac villous atrophy should be considered in “nonresponsive celiac disease” and causes include peptic inflammation, Giardia , Helicobacter infection, and Crohn disease. Histologic grades that fall short of villous atrophy, ie, Marsh grade 1 and 2, are a gray area. There is some evidence that suggests this situation should be managed as celiac disease with villous atrophy. One group randomized 23 patients with positive EMA, compatible HLA status, and Marsh grade 1 or 2 changes to receive either normal diet or GFD. Those on GFD had improvement symptomatically, serologically, and histologically. Those on normal diet showed persistence or worsening of all parameters. This finding is supported by others. However, caution should be exercised because these lesser changes are not uncommon and are not always attributable to celiac disease. Other causes include drugs, immune dysregulation, and infection.
Diarrhea in celiac disease
Although it is increasingly recognized that celiac disease can present without gastrointestinal symptoms, diarrhea remains a common presenting complaint. One large survey included 2681 adults with biopsy-proven celiac disease. In this study, the most common, self-reported presenting symptoms included abdominal pain (83%), diarrhea (76%), and weight loss (69%). Screening populations could be expected to have fewer symptoms than health care–seeking individuals, although the astute physician may identify symptoms and signs. In one primary care screening study, 976 patients were investigated and celiac disease was diagnosed in 22 patients. Of these, 6 of 22 had unexplained chronic diarrhea.
Nonresponsive Celiac Disease
Nonresponsive celiac disease (NRCD) has historically been described as failure to respond to a GFD. This can be primary NRCD (ie, an individual who never responded to a GFD) or a subsequent recurrence of symptoms after 1 year (secondary NRCD). Contemporary clinical studies suggest that NRCD occurs in about 17% to 30%. Most cases of NRCD are related to either inadvertent ingestion of tiny, but clinically significant amounts of gluten or nonadherence.
In the patient with NRCD, it is important to challenge the initial diagnosis of celiac disease. In one reported series of 55 patients referred for NRCD, the original diagnosis of celiac disease was disproved in 6. All previous diagnostic investigations including the serology and duodenal biopsies should all be reviewed. Biopsies should be reviewed by a specialist gastrointestinal pathologist. Supportive evidence should be sought in the form of compatible HLA status (DQ2 or DQ8), a positive family history of celiac disease, or functional hyposplenism.
Gluten Exposure
Adherence to a GFD is estimated to be in the order of 42% to 91% based on self-reporting. The most common reason for NRCD is continued gluten exposure. In one study of patients with celiac disease and persisting symptoms, 25 of 49 were identified as having gluten contamination responsible for their ongoing clinical and histologic signs. Inadvertent ingestion usually occurred in the form of commercially packaged cereals derived from corn or rice that had contained malted barley. Adherence is best assessed by a skilled dietician.
Associated Conditions
There are other causes of chronic diarrhea in patients with celiac disease who are adherent to a GFD. Diagnoses associated with celiac disease include microscopic colitis, pancreatic insufficiency, irritable bowel syndrome (IBS), small bowel bacterial overgrowth, thyroid dysfunction, and secondary intolerances (owing to mucosal surface damage, for example, lactose or fructose intolerance). Microscopic colitis is more common in patients with celiac disease than in controls without celiac disease. One recent large population-based study found an incidence of microscopic colitis in celiac disease 50 times that in the general population. The association most often occurred in middle-aged women. Diagnosis requires pan-colonic biopsies. Pancreatic abnormalities are associated with celiac disease. One study of patients with celiac disease and chronic diarrhea found that almost one-third (20/666) had low fecal pancreatic elastase levels. Furthermore, pancreatic supplementation conferred some benefit with a reduction in stool frequency.
IBS can mimic the symptoms of celiac disease and it is recommended that patients presenting with IBS are tested for celiac disease. Small bowel bacterial overgrowth is recognized as a cause of persisting symptoms in celiac disease. In one study of 15 patients with persisting symptoms, 10 showed small intestinal bacterial overgrowth by lactulose breath test. When these patients were treated with antibiotics (rifaximin) after 1 month, they were symptom free ; however, antibiotic therapy does not always improve symptoms and breath tests may not reliably identify those who will respond. Adult autoimmune enteropathy is recognized as a rare cause of villous atrophy. Patients have severe enteropathy unresponsive to any exclusion diet and are positive for enterocyte antibodies.
Refractory Celiac Disease
The normal small intestinal intraepithelial lymphocyte (IEL) population is composed of approximately 80% to 85% CD8+ T-cell receptor (TCR)αβ cells and 15% CD8+TCRγδ cells. In uncomplicated celiac disease, IELs express CD3+ and CD8+ (T suppressor/cytotoxic phenotype) and there is an increase in γδ T cells. Celiac disease may be regarded as refractory (RCD) when symptoms persist (primary) or recur (secondary) despite the adherence to a strict GFD and when other causes have been excluded. RCD is subdivided into types I and II with a phenotypically normal or aberrant intraepithelial T-cell population respectively. This is determined by using polymerase chain reaction analysis for the TCR gene rearrangement. Quantification in percentage terms is used as a means of differentiating RCD I from RCD II. Patients with RCD I have less than 10% of aberrant (clonal) T cells on duodenal biopsy. This differentiation helps in prognostication. RCD I is more likely to respond to immunosuppression, whereas RCD II seems largely resistant to treatment, and transition to enteropathy-associated T-cell lymphoma (EATL) is common. Although ulcerative jejunitis and EATL remain rare complications of celiac disease overall, they are relatively common in a subset of patients with RCD II. In one study of 93 patients with RCD, 43 had RCD I and none had celiac-related mortality at 5 years. In the group with RCD II (n = 50), the overall 5-year survival was 58%, falling to 8% in the 26 (52%) who developed EATL. A recent study has demonstrated that the aberrant immunophenotype and monoclonality may be transiently detected in patients with celiac disease who are not adherent, however; thus, it is important not just to take a snapshot but to have repeat testing.
Typically, RCD presents with severe malnutrition, malabsorption, hypoalbuminemia, and weight loss. Weight loss can be predictive of refractory CD. HLADQ2 homozygous patients appear more likely to develop secondary EATL or de novo EATL. Therapeutic options are limited but there is some evidence that azathioprine and prednisolone (or budesonide) may confer benefit in RCD I. For type II refractory disease and/or EATL, many other treatments, including cyclosporine, interleukin-10, elemental diet, alemtuzumab, cladribine, CHOP (a combination of doxorubicin, cyclophosphamide, vincristine, and prednisolone), and autologous stem cell transplantation have been described in case series. It is beyond the scope of this review to give further detail.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





