Fig. 39.1
Large perineal defect demonstrating vast dead space and exposed viscera
Primary closure of large perineal defects can be associated with unacceptable wound-healing complications ranging as high as 65 % [2–4]. Other common surgical complications include hematoma, seroma, abdominal and/or perineal hernia, fistulization, and nonhealing wounds [1]. Such complications can lengthen hospital stays, decrease mobility, prolong time to adjuvant therapy, and increase patient morbidity.
In an attempt to mitigate the occurrence of such complications, many plastic and reconstructive surgeons advocate the use of a pedicled, vascularized soft-tissue flap coverage to close large perineal defects [3, 4]. Utilizing a vascularized, soft-tissue flap will assist in obliterating dead space and this has shown to improve wound-healing outcomes by decreasing incisional tension while increasing vascular supply, oxygenation, and delivery of cytokines and growth factors. In the current chapter, we aim to provide a comprehensive overview of the various reconstructive options for perineal defects to educate the reader on how to prevent as well as treat perineal wounds in an effort to maximize patient outcomes in these challenging cases.
Preoperative Evaluation
The physiologic status of the patient must be considered and balanced with the overall reconstructive plan. The long-term prognosis of the patient must also be taken into account as well. Reconstruction to improve quality of life should be considered even in a palliative scenario. If possible, an accurate assessment of the wound or planned surgical site should be performed in the preoperative setting and may be facilitated by an examination under anesthesia.
Medical Comorbidities
A thorough review of the patient’s past medical history should be performed to risk-stratify each patient using conditions that increase the relative risk of infection and/or wound-healing problems. Smoking has been shown to affect microcirculatory blood flow and soft-tissue healing and should be discontinued a minimum of 4 weeks prior to surgical intervention if possible [5]. Patients undergoing major reconstructive efforts require optimization of nutritional parameters prior to intervention [6–8]. If enteral feeding is not possible preoperatively, postoperative enteral or parenteral nutrition should be administered with protein supplementation.
Radiation Therapy
Radiation therapy induces tissue injury through changes in the microcirculation of the defect and its surrounding areas, leading to decreased perfusion and impaired wound healing [4]. The reconstructive surgeon should be cognizant of the timing, dosage, and location of any prior or planned radiation and not limit debridement of radiated tissue. Additionally, the surgeon should utilize tissue outside the field of radiation for flap reconstruction.
Chemotherapy
Neoadjuvant chemotherapy can significantly impair wound healing and should be considered when selecting reconstructive options. The plastic and reconstructive surgeon should work closely with the medical oncology team to determine if and when the patient will require adjuvant chemotherapy, as wound-healing complications can potentially delay onset to therapy.
Imaging
Preoperative evaluation with imaging modalities such as computed tomography (CT) and/or magnetic resonance imaging (MRI) could be obtained to evaluate the integrity of the surrounding soft tissue and vascular anatomy. While these adjuncts are not essential, they can assist the surgeon in surgical planning.
Timing of Reconstruction
The timing of perineal reconstruction is most often dictated by the status of the tumor and surgical margins. Certain factors such as advanced age, multiple comorbidities, or the need for loco-regional control with adjuvant radiotherapy must also be considered in the timing. Primary reconstruction carries a significantly decreased rate of wound-healing complications and is preferred to delayed reconstruction in defects located in other areas of the body [9, 10]. Delayed reconstruction, while not optimal, is occasionally unavoidable for defects with extensive soft-tissue deficits or patient instability. If a patient requires delayed reconstruction, a negative pressure closure device is the preferred temporizing measure until definitive reconstruction can be performed at a later time.
Classification of Defect
Acquired perineal defects should be classified according to what structures are missing and/or compromised. This will allow the reconstructive surgeon to employ the correct reconstructive modality in an effort to replace damaged tissue with tissue that is most similar to that which is missing. The classification of perineal defects is listed in Table 39.1.
Table 39.1
Classification of perineal defects by anatomic location
Anatomic structure(s) involved | Missing tissue components |
|---|---|
Vaginal vault | S, MS, ST |
Vulvoperineal surface | MS, ST |
Scrotum | S, ST |
Penis | S, MS, ST |
Perineum and pelvic support musculature | S, ST (extensive) |
Sacrum/pelvic rim | S, ST, +/− osseous involvement |
The size of the defect and types of missing tissue should be assessed for viability. Potential flap donor sites should be evaluated for adequate rotational length. If there is a potential for microvascular free tissue transfer, recipient donor vessels should be evaluated for patency. If these vessels are unavailable or greater length is required, arteriovenous loop can be created if needed. However, most perineal defects can be reconstructed with the use of local flaps typically from the abdominal wall, thigh, or buttock region.
Reconstructive Surgical Tenants
The core principles underlying reconstructive algorithms used by plastic and reconstructive surgeons are to progress from simple to more complex reconstructions on the basis of the specific wound requirements. The goal for each reconstruction is to provide a tension-free closure that obliterates all dead space which replaces the defect with tissue that is most similar to what is missing. The adage is “like with like.”
Local tissue flaps enable surgeons to reconstruct soft-tissue defects with similar tissue from an adjacent location. Axial pattern flaps are based on named blood vessels and are the mainstay of perineal reconstruction. Axial pattern flaps can be fasciocutaneous (deep muscle fascia with overlying skin), myocutaneous (muscle with skin), and myofasciocutaneous (muscle with deep fascia and overlying skin), which will enable reconstructive surgeons to repair defects with tissue that is similar to the resected tissue.
Microvascular free tissue transfer involves harvesting a tissue construct and its named blood supply from a distant region of the body and placing it into a defect using microvascular anastomosis between the flap’s donor vessels and the patient’s recipient vessels. Most cases are performed under magnification provided by a surgical microscope. The decision to use a particular flap is based on the requirements for replacing missing skin, adipose tissue, fascia, and muscle. The primary advantage of microvascular free tissue transfer is that tissue of a quality similar to that of the resected tissue can be moved from a remote part of the body, thereby enabling optimal aesthetic and functional outcomes. This also allows irradiated or infected tissue to be removed and replaced with soft, pliable, and vascularized tissue from a different part of the body, outside of the field of injury. Drawbacks of free tissue transfer are related to donor site morbidity and the potential for longer operative times.
Adjuncts to Flap Surgery
Negative Pressure Wound Therapy
Negative pressure-assisted closure can provide for temporary coverage in soft-tissue perineal defects when definitive reconstruction is either delayed or not required. When utilized appropriately, this device can promote neo-vascularization, decrease edema, and increase local granulation tissue as well as providing contractile force at wound edges [11]. This modality is often used to prepare the wound bed for definitive reconstruction with soft-tissue flaps in a delayed fashion if immediate surgical intervention is not possible. Additionally, it can also be used to promote healing by secondary intention in partial-thickness defects.
Tissue Expansion
Tissue expansion is a process in which an inflatable prosthetic implant with a silicone shell is used to expand local and regional tissues so that they can eventually be advanced into the wound in a delayed fashion. The inflatable implant is inserted at the time of tumor extirpation or during a second procedure. At subsequent office visits, saline is injected through an integrated or remote port to gradually expand the implant. Once the tissue has been sufficiently expanded, it can be advanced into the defect in a second surgical procedure. Because tissue expansion takes time, the method is not feasible for immediate perineal reconstruction that requires immediate coverage of hollow viscous or neurovascular structures. Risks of tissue expansion include infection, extrusion, and rupture of the implant [12]. Additionally, the sequential expansion of the prosthesis can be uncomfortable to the patient and requires multiple office visits to obtain satisfactory expansion.
Biologic Tissue Matrices
Commercially available biologic tissue matrices (BTMs) currently come from five different sources: human dermis, porcine dermis, porcine small intestinal submucosa, bovine dermis, and bovine pericardium [13]. BTMs claim to induce early revascularization capacity in an effort to provide soft-tissue coverage and resist infection. For perineal reconstruction, BTMs can be used in a multitude of capacities including the creation of pelvic diaphragms to prevent visceral herniation into low perineal defects. Additionally, BTMs can be used to reinforce abdominal site donor defects in an effort to decrease bulge and hernia formation in the face or prior irradiation or concurrent ostomy placement.
Characterization of Axial Pattern Flaps (Table 39.2)
Table 39.2
Commonly employed pedicled flap options for perineal reconstruction
Flap name | Blood supply | Area of use |
|---|---|---|
Rectus abdominis | Inferior epigastric artery | Total perineal reconstruction/posterior vaginal wall |
Gracilis | Medial circumflex femoral artery | Smaller perineal defects |
Gluteus maximus | Superior gluteal artery | Posterior/inferior perineal defects |
Pudendal | Posterior labial artery | Vaginal vault defects |
Anteriolateral thigh | Descending branch of lateral circumflex femoral artery | Total perineal reconstruction |
Rectus Abdominis Muscle
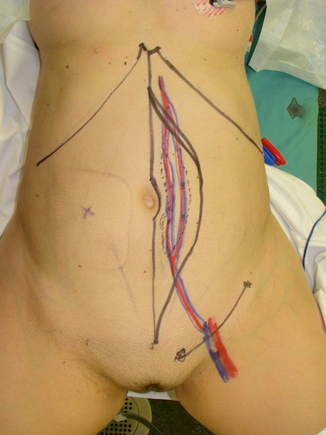
The pedicled vertical rectus abdominis (VRAM) flap is a versatile flap based on the deep inferior epigastric system. The flap can be harvested as a muscle-only, myocutaneous flap, or perforator flap with a large skin paddle. All but the largest of myocutaneous flaps can still allow for primary closure of the donor site. However, as previously mentioned, synthetic or BTM reinforcement may be required to prevent subsequent complications. The flap has a robust vascularity and abundant soft-tissue bulk that can be used to obliterate the vast amounts of dead space seen with large APR defects [14–17]. Butler et al. demonstrated that despite the overall complication rate not being significantly different between primary closure and VRAM flap reconstruction, patients who underwent VRAM reconstruction experienced significantly lower incidences of perineal abscesses (9 vs. 37 %) and major wound dehiscence (9 vs. 30 %) (Fig. 39.2) [17].
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








