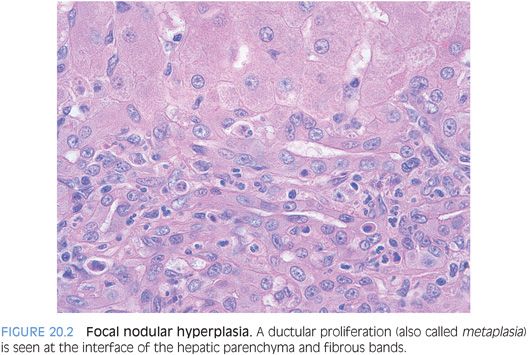
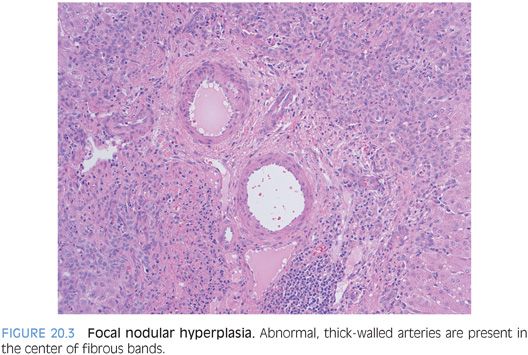
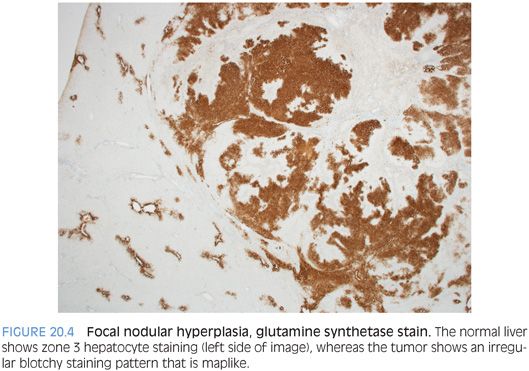
A subset of focal nodular hyperplasias can have ballooning and Mallory hyaline (eFig. 20.3). Fatty change is less common, but when fat is present along with the balloon cells and Mallory hyaline, the findings can mimic steatohepatitic hepatocellular carcinoma. The overall architecture and the cytologic features can usually allow distinction of these two possibilities on hematoxylin and eosin (H&E). In challenging cases, special stains for glutamine synthetase can be helpful. A maplike staining pattern favors focal nodular hyperplasia. Diffuse or negative staining would be more consistent with a well-differentiated hepatocellular carcinoma than a focal nodular hyperplasia. Overall, approximately half of well-differentiated hepatocellular carcinomas are diffusely positive glypican 3. Other stains that can be helpful include reticulin, Ki-67, and glypican 3.
The background liver should be noncirrhotic. Cirrhotic livers frequently have vascular shunting and sometimes develop nodules that resemble focal nodular hyperplasia. This has led to the suggestion to use the term focal nodular hyperplasia in this context. However, any upside to expanding the definition of focal nodular hyperplasia to include lesions in cirrhotic livers is offset by the greater risk of obscuring the biology and clinical correlates of traditional focal nodular hyperplasias.
A large body of published literature emphasizes that pathologists confidently diagnose focal nodular hyperplasia on needle biopsy in only approximately 25% to 50% of cases. In part, this reflects sampling issues, and multiple passes will increase the likelihood of a nonequivocating diagnosis. In addition, this “lack of diagnosis” is in part because a diagnosis of focal nodular hyperplasia is often made only after correlating the imaging and histologic findings. Thus, a pathologist may say the findings are consistent with focal nodular hyperplasia after correlation with imaging studies. Finally, there is an increasing trend for only lesions with atypical imaging findings to be referred for biopsy and these cases are also less likely to have typical histologic findings on a needle biopsy. Immunostaining for glutamine synthetase can help clarify many of these more challenging cases.
Differential
The differential for focal nodular hyperplasia includes inflammatory adenomas (also called telangiectatic adenomas). Elements that favor a focal nodular hyperplasia include the fibrous bands, abnormal thick-walled vessels, and a ductular proliferation that is typically somewhat patchy and located within the fibrous bands. The inflammatory adenomas also have ductular-like proliferations, but the ductular-like proliferations are located in faux portal tracts and not fibrous bands. Finally, the differential includes focal nodular hyperplasia–like areas that develop around a mass lesion that has caused vascular shunting. Neoplasms that can cause this rim of focal nodular hyperplasia–like changes include hemangiomas, fibrolamellar carcinoma,3 and metastatic neuroendocrine carcinomas. In these cases, this reactive rim of changes can be indistinguishable from an otherwise ordinary focal nodular hyperplasia on needle biopsy.
Immunostains
Glutamine synthetase normally stains a thin rim of hepatocytes around the central veins. In contrast, staining in focal nodular hyperplasias will show an irregular “maplike” pattern (Fig. 20.4) and can significantly increase the confidence in making the diagnosis over that of H&E alone.4 A diffuse staining pattern can be seen in both hepatic adenomas and hepatocellular carcinoma and does not suggest focal nodular hyperplasia. Other useful stains can include a cytokeratin stain to highlight the proliferating bile ductules, a copper stain to highlight the cholate stasis, and a reticulin stain to help rule out malignancy.
MACROREGENERATIVE NODULE
Definition
Macroregenerative nodule is a benign nodule in a cirrhotic liver whose size makes it stand out grossly from the background liver. A diagnosis of a macroregenerative nodule is best made on a resection or wedge biopsy and not a needle biopsy. The frequency is approximately 15% in explanted livers. Macroregenerative nodules can also be seen in noncirrhotic livers that have undergone extensive liver parenchymal loss with large areas of panacinar collapse, such as fulminant hepatitis.
The basic notion of this lesion is simple: a nodule that stands out as being significantly bigger than its peers in a cirrhotic liver (usually the nodule is larger than 15 mm) but histologically shows no or minimally cytologic atypia and no loss of reticulin or other features to suggest malignancy. However, this simplicity has been lost to a large degree because confusing and competing nomenclature and definitions have been proposed in the literature. Nonetheless, the basic notion and terminology as outlined earlier is still quite helpful, especially with wedge or resection specimens. Histologically, the hepatocytes within macroregenerative nodules look essentially the same as hepatocytes located outside the nodule. There often are portal tracts within the macroregenerative nodule.
DYSPLASTIC NODULE
Definition
Dysplastic nodule is a cirrhotic nodule that is distinct from the background cirrhosis and has some but not all features of malignancy. The notion of dysplastic nodules is also simple in theory but has been difficult to reliably define histologically. The basic notion is that a nodule shows atypical features that are clearly more than that of a regenerative nodule but less than that of hepatocellular carcinoma. These atypical features may include areas of pseudogland formation (less helpful in cholestatic livers), increased although generally still mild nuclear atypia, intraparenchymal arterioles, or complex architecture with a “nodule within a nodule” appearance. Portal tracts are often present within the dysplastic nodules. Dysplastic nodules may or may not have large cell change or small cell change. There should be no loss of reticulin and no or very few mitotic figures. α-Fetoprotein (AFP) immunohistochemistry is negative. β-Catenin immunostaining is negative for nuclear accumulation. In research studies, they can be further divided into high-grade and low-grade dysplastic nodules, but the efficacy and value of that division remain unclear, especially on needle biopsies performed for routine surgical pathology. In addition, many liver pathologists (including the author) believe the distinction between a low-grade dysplastic nodule and a macroregenerative nodule is fairly arbitrary using currently available methods.
HEPATIC ADENOMA
Definition
Hepatic adenoma is a benign neoplasm composed of hepatocytes. Synonyms include hepatocellular adenoma and liver adenoma. As you read the literature, be aware that some authors use the term adenoma to refer to any well-differentiated neoplasm, including those that develop in men with chronic viral hepatitis and advanced fibrosis. The term adenoma, as used for these types of cases is essentially a synonym for “very well-differentiated hepatic tumor.” For this reason, some adenomas described in the literature, especially some of the β-catenin–activated adenomas, would be called well-differentiated hepatocellular carcinomas in other centers.
Clinical Findings
Hepatic adenomas occur primarily in young to middle-aged women with a history of oral contraceptive use. However, any population with estrogen use for medical purposes can develop adenomas. Likewise, exogenous androgen use is a recognized risk factor for hepatic adenomas. In cases due to exogenous sex hormone exposure, the background livers are without other liver disease and show no fibrosis. Other recognized risk factors for hepatic adenomas include glycogen storage disorders, especially type I and type III.5 Adenomas have also been reported with glycogen storage disease type IV.6 The adenomas in the setting of glycogen storage disease are more common in men than women.5
Histologic Findings
Hepatic adenomas are well-differentiated hepatic neoplasms. Hepatic adenomas should have essentially no cytologic atypia and essentially no mitotic activity (Fig. 20.5). The adenomas are generally not encapsulated but in some areas may have a network of thin-walled vessels at the interface of the tumor and nontumor that can grossly resemble a capsule. Hepatic adenomas can be so well differentiated that it may be difficult at high power to tell if you are in tumor or nontumor. In these cases, move to low power and use the loss of portal tracts to tell you when you are in the adenoma. Finding aberrant lobular arteries can also be helpful (eFig. 20.4). Some adenomas can have fatty change, but in these cases, it tends to be just steatosis, without the Mallory hyaline, balloon cells, inflammation, or intratumoral fibrosis that make up the histologic pattern of steatohepatitic variant of hepatocellular carcinoma.
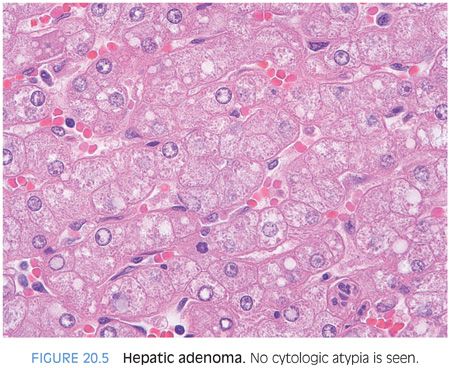
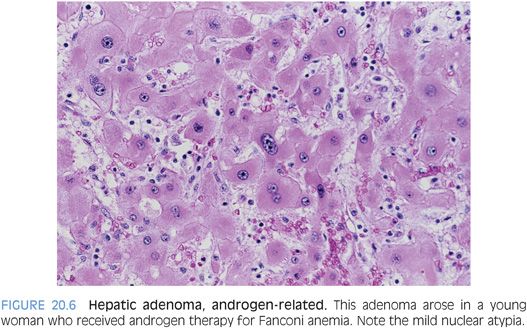
HEPATIC ADENOMA VERSUS WELL-DIFFERENTIATED HEPATOCELLULAR CARCINOMA. How do you separate a well-differentiated hepatocellular carcinoma from a hepatic adenoma? Hepatic adenomas should have no cytologic atypia, inconspicuous nucleoli, no mitotic activity, and a low (less than 1%) Ki-67 proliferative rate. One exception is that there can be increased proliferation near areas of tumor necrosis (eFig. 20.5). Hepatic adenomas are rarely cholestatic and rarely, if ever, have a pseudoacinar growth patterns. They generally do not have cytoplasmic inclusions (eFigs. 20.6 and 20.7). As one exception to cytologic atypia, androgen-related hepatic adenomas can show mild cytologic atypia (Fig. 20.6, eFig. 20.8).
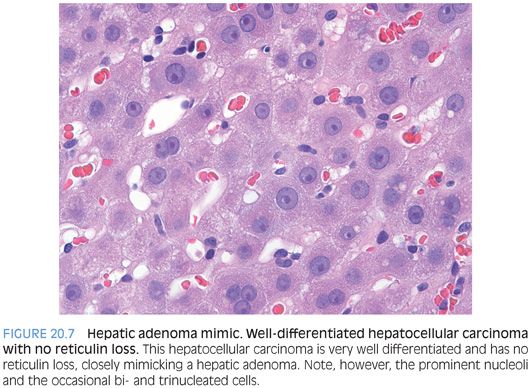
Hepatic adenomas should have essentially no reticulin loss. Reticulin staining is a very helpful tool, but there are several caveats. First, definite reticulin loss should push the diagnosis to hepatocellular carcinoma, but the significance of focal equivocal reticulin loss is less clear. Also, the reticulin framework can be artificially disrupted near areas of hemorrhage. In addition, well-differentiated hepatic tumors with abundant fatty change (such as hepatocyte nuclear factor 1α [HNF1α]–mutated adenoma) can also have small foci of reticulin reduction due to the steatosis. Finally, very rare well-differentiated hepatocellular carcinomas do not show reticulin loss, especially on biopsy, so reticulin interpretation should take place in the context of other histologic findings (Fig. 20.7). These caveats are important but do not diminish the important role of the reticulin stain when evaluating well-differentiated hepatic tumors. Outside of very rare exceptions, all hepatic tumors that behave aggressively have recognizable reticulin loss.
If the clinical background is unknown or is atypical for a hepatic adenoma, then approach the diagnosis with added care. If there are any clinical or histologic findings that are not a good fit for a hepatic adenoma on the liver biopsy, then its best to use a term such as well–differentiated hepatic neoplasm and give the differential in a note. Relevant to this, there is a small subset of well-differentiated hepatocellular neoplasms (<5%) that are difficult to classify even when fully resected. They have definite but very mild cytologic or architectural atypia yet do not have the full features of hepatocellular carcinoma. Currently, there is no definitive way to determine if these types of cases are atypical adenomas or well-differentiated hepatocellular carcinoma. If you send these cases to expert hepatic pathologist, you will get different opinions. The good news is that these cases are cured by resection. Given that rare cases are difficult to classify when fully resected, it should be fully anticipated that on biopsy, occasional cases are best approached by a diagnosis of a “well-differentiated hepatic neoplasm” followed by a description and prioritized differential in the comment.
Hepatocellular Adenoma Subtypes
Molecular studies have divided hepatic adenomas into four subtypes. A fifth type is the pigmented adenoma. Molecular subtyping represents a major advance in the understanding of the biology of hepatic adenomas and has been largely driven by important studies over the past decade from Bordeaux and Paris, France, where collaborative efforts by pathologists, radiologists, hepatologists, and molecular biologists have led to numerous key observations. The molecular-based classification is summarized in Table 20.1. When using this table, please note that many of the individual histologic findings are overlapping between subtypes and can be variable within any single subtype. Thus, it is the overall pattern, and not any single finding, that should be used to make your diagnosis.
Subtyping hepatic adenomas using the features in Table 20.1 should only be performed after you make a diagnosis of hepatic adenoma. If you still are uncertain whether a tumor is a hepatic adenoma or a well-differentiated hepatocellular carcinoma, do not try to subtype the adenoma. Make the diagnosis first then subtype. Hepatocellular carcinomas can have immunostain findings that mimic the staining patterns found in hepatocellular adenomas. This point bears reemphasis because it is persistent source of diagnostic confusion: Make the diagnosis first then subtype as appropriate.
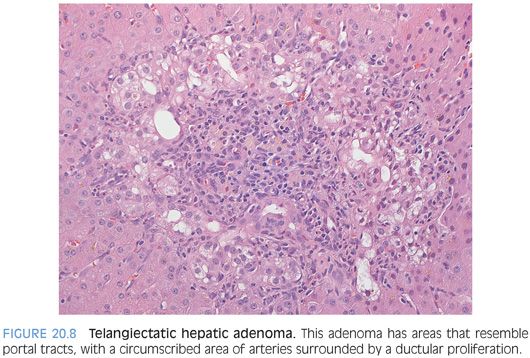
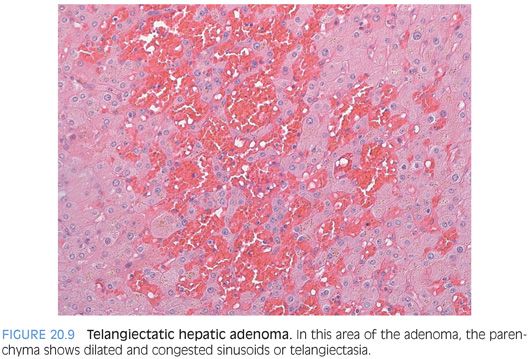
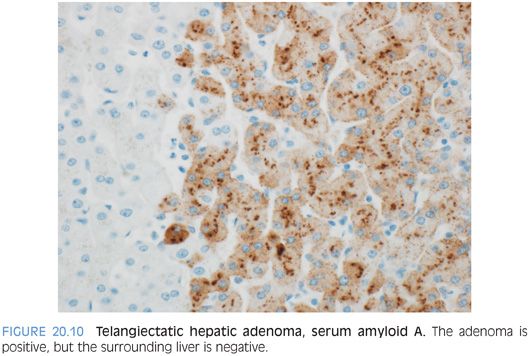
The most histologically distinct subtype are the inflammatory (also called telangiectatic) adenomas and the HNF1α-mutated adenomas. The inflammatory adenomas have distinctive faux portal tracts with vessels embedded in a fibrous stroma surrounded by a ductular-like reaction (Fig. 20.8, eFigs. 20.9 to 20.11). They also typically have dilated (i.e., telangiectatic) and congested sinusoids (Fig. 20.9), occasionally with large aberrant arteries in the parenchyma. Many but not all inflammatory adenomas have inflammatory infiltrates, some of which can be striking. The inflammation is usually in the portal tract–like areas. Some cases can also show marked fatty change (eFig. 20.12). Immunostains for serum amyloid A are positive in the tumor but not the adjacent liver (Fig. 20.10).
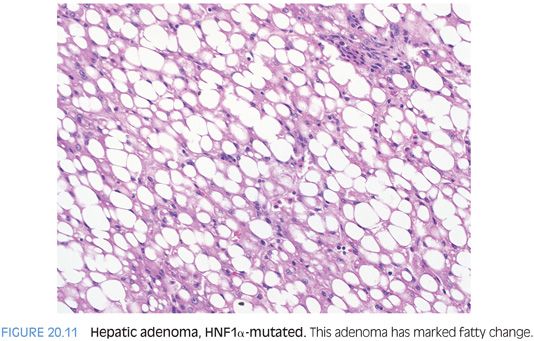
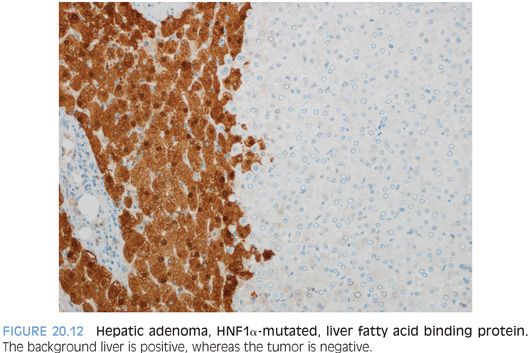
For HNF1α-mutated adenomas, the most striking feature is steatosis, which tends to be moderate to marked and diffuse but lacks significant inflammation, balloon cells, or intratumoral fibrosis (Fig. 20.11). Rare HNF1α-mutated adenomas will have no fatty change and are recognized only by their loss of liver fatty acid binding protein expression on immunostaining (Fig. 20.12). The differential for HNF1α-mutated adenomas with fatty change is primarily that of the steatohepatitic variant of hepatocellular carcinoma. In contrast to the adenoma, the steatohepatitic variant of hepatocellular carcinoma typically has more variable-sized fat droplets, balloon cells, often Mallory hyaline, and intratumoral fibrosis. The hepatocellular carcinoma should also have increased mitoses, cytologic atypia, reticulin loss, and can be glypican 3–positive. Both can have mild tumor inflammation.
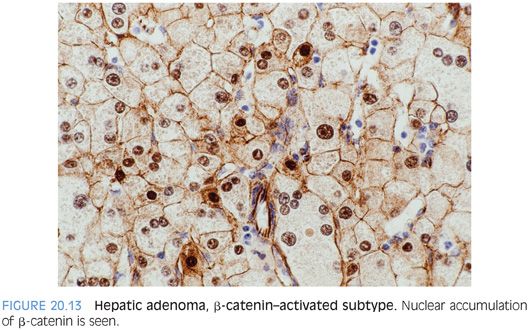
Some hepatic adenomas will show nuclear accumulation of β-catenin (Fig. 20.13). If the adenoma shows features of an inflammatory adenoma or an HNF1α-mutated adenoma, then the diagnosis is based on those findings; for example, an inflammatory adenoma may also have β-catenin nuclear positivity and would then be called an inflammatory adenoma with β-catenin activation. If the adenoma has features of neither an inflammatory adenoma nor an HNF1α-mutated adenoma but shows β-catenin nuclear staining or diffuse glutamine synthetase staining, then the adenoma is best classified as a β-catenin–activated adenoma. The diagnosis of adenoma, however, should come before the subclassification using immunostains. Both β-catenin and glutamine synthetase are positive in hepatocellular carcinoma. An additional subset of adenomas has none of these features and is put into the “unclassified hepatic adenoma” category.
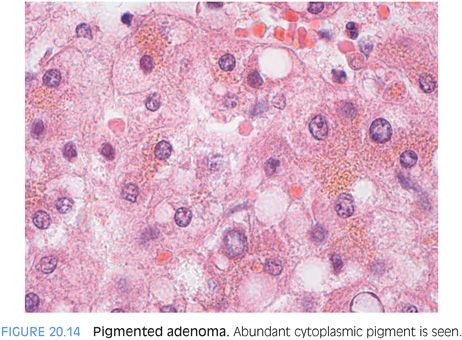
A final subtype is pigmented hepatic adenomas (Fig. 20.14). There are very few reports of this rare subtype, so the available descriptions of the range of clinical and pathologic findings are likely incomplete. They have been reported almost exclusively in males7,8 and have abundant brown cytoplasmic pigment that resembles Dubin-Johnson pigment. They can demonstrate mild cytologic atypia and in some cases can progress to frank malignancy.8 In fact, many cases truly straddle the histologic fence between adenoma and hepatocellular carcinoma, and sometimes, they appear in the literature as either.
Is subtyping of hepatic adenomas necessary for patient care? Currently, there is no convincing data that management of the adenoma should be based on the subtype. This may change in the future of course but has not changed yet. Instead, management is based on tumor size, clinical findings, and atypical radiologic or histologic findings. β-Catenin–activated adenomas have been associated with an increased risk for malignancy in the literature. However, based on the author’s experience as well as a prudent interpretation of the published literature, an increased risk for malignancy appears most likely to be present only when the β-catenin nuclear staining is also accompanied by cytologic or architectural atypia. β-Catenin nuclear staining alone in an otherwise typical adenoma appears less likely to have increased risk above that of ordinary adenomas.
Malignant Transformation of Hepatic Adenomas
The overall risk of malignant transformation has not been well defined, but approximately 10% of resected hepatic adenomas reported in the literature have underwent malignant transformation.9 Many cases of malignancy do not arise in β-catenin–activated adenomas. However, in most cases of malignant transformation, the adenomas were 5 cm or more in greatest dimension. Thus, current management guidelines are based on size and the general recommendation is for adenomas greater than 5 cm to be resected. Histologic subtype is not a major factor in current management guidelines, although that might change with time as more data accumulates.
Immunostains
Hepatic adenomas should have no reticulin loss and should have very low proliferative rates on Ki-67 immunostaining. One caveat is that the tumor cells near areas of necrosis can show a mildly increased proliferative rate. Hepatic adenomas should be glypican 3–negative. CD34 immunostaining typically shows patchy sinusoidal staining in hepatic adenomas, versus diffuse staining in hepatocellular carcinoma, but does not reliably distinguish adenomas from hepatocellular carcinoma on biopsy. Additional immunostains for subtyping are included in Table 20.1.
HEPATOCELLULAR CARCINOMA: GENERAL PRINCIPLES
Definition
Hepatocellular carcinoma (HCC) is a malignant liver tumor that shows hepatocyte differentiation.
Clinical Findings
Older men with chronic liver disease are at the highest risk for HCC. The incidence increases steadily after the age of 40 years, and the average age is approximately 65 years. Overall, the most important risk factor is cirrhosis from any cause. Worldwide, chronic hepatitis B is the most common risk factor, but in the United States, much of Europe, and Japan, chronic hepatitis C is the most common risk factor. The metabolic syndrome is both a contributing factor for carcinoma in patients with chronic viral hepatitis C10 and an independent risk factor for HCC.11 Other important causes include alcohol-related liver disease and hemochromatosis. Cirrhosis from chronic biliary tract disease can also be a risk factor.
Of note, 15% to 20% of HCCs arise in noncirrhotic livers.12 Of these cases, approximately 50% will have chronic liver disease that is thought to have caused the cancer, despite the lack of fibrosis. Chronic hepatitis B,13 chronic hepatitis C,14 HFE-related hemochromatosis,15 fatty liver disease,16,17 and malignant transformation of hepatic adenomas9 have all been linked to HCC in noncirrhotic livers. No cause is identified in the remaining subset, despite full clinical and pathologic evaluation.
How to Make the Diagnosis of Hepatocellular Carcinoma
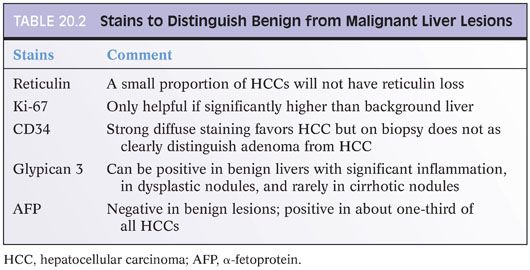
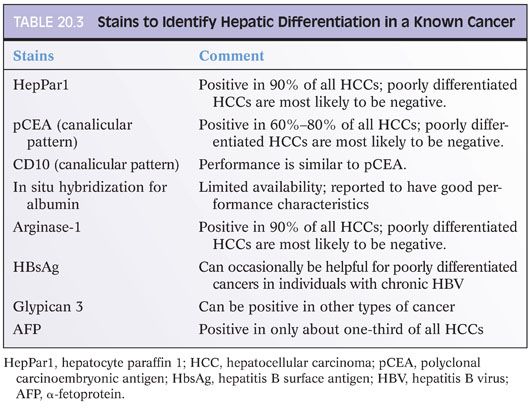
Most HCCs are recognizable on H&E stains, but many require confirmation with special stains. In the process of choosing which special stains to perform, a useful first step is to ask yourself this question: Is the tissue clearly liver parenchyma, but you are not sure if it is benign or malignant? Or alternatively, are you sure there is cancer on the biopsy, but you are not sure if the cancer is hepatocellular or metastatic? The stains you should get will depend substantially on the answer to this question (Tables 20.2 and 20.3). If the tissue in question is clearly liver, then the differential is typically that of a regenerative nodule, dysplastic nodule, focal nodular hyperplasia, hepatic adenoma, or HCC. Stains that will help with this differential are shown in Table 20.2. In contrast, if the tumor is clearly malignant, but you are not sure if it is metastatic, then stains for hepatic differentiation (shown in Table 20.3) will help you make the diagnosis. A common misstep is not making this distinction up front, which often leads to unnecessary stains and sometimes to exhaustion of the block before a full diagnosis can be made.
Hepatocellular Grading
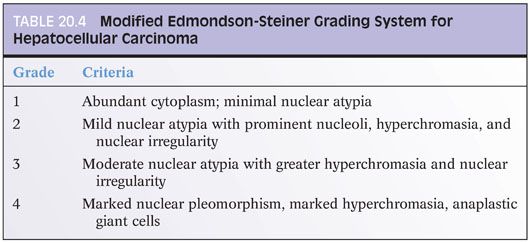
Hepatocellular grading has not been standardized to a completely satisfactorily level. Research studies commonly use the modified Edmondson-Steiner grading system, which is summarized in Table 20.4.18 Two or more nuclear grades can be present in which case the tumor is classified according to the worse nuclear grade. Grading for clinical purposes tends to be more of a gestalt and thus not terribly reproducible among centers. One approach is as follows: well differentiated—the tissue is clearly liver, you need stains to make sure it is cancer; moderately differentiated—on H&E, the tissue is clearly cancer and hepatocellular differentiation is morphologically evident; or poorly differentiated—on H&E, the tissue is clearly cancer, but you really needed immunostains to be sure it had hepatocellular differentiation.
Background Liver
Do not forget the importance of examining the background liver for active injury and for fibrosis stage. To do this, a section(s) should be taken as far away from the tumor as possible, ideally at least 1 cm. With biopsy specimens, this is of course problematic unless a separate biopsy was intentionally taken away from the mass lesion. If not, estimates of fat, inflammation, cholestasis, and fibrosis can be strongly influenced by the nearby tumor mass and may not reflect the background liver. Also, with resection specimens, avoid the cauterized resection edges as much as possible.
Prognosis
The single most important prognostic factor is tumor resectability. Other prognostic findings include age (the younger, the better), gender (women, better), and the presence or absence (better) of cirrhosis in the background liver. Comorbid conditions such as heart disease also strongly influence survival.
Pathology findings that influence survival include tumor size, tumor number, and the presence of angiolymphatic invasion (eFigs. 20.13 and 20.14). These combined factors are key elements in most tumor staging systems. For angiolymphatic invasion, large vessel invasion is defined as vessels that are large enough to identify on imaging or gross examination that are positive for tumor. Large vessel invasion has a worse prognosis than small vessel invasion, which is recognized only on microscopy. Tumor grade also influences prognosis, as do morphologic variants discussed in the following texts.
Histologic Findings
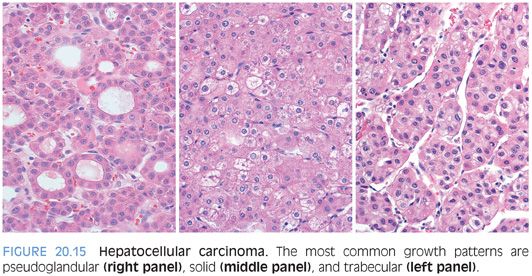
Cytologic findings and architectural growth patterns show considerable variation in HCCs (eFigs. 20.15 to 20.28). Well-differentiated HCCs have abundant pink cytoplasm and round nuclei with dispersed chromatin and variably prominent nucleoli. Aberrant arteries (because they are located in the lobules and not the portal tracts) are not specific but can be a useful finding in separating well-differentiated tumors from the background liver (eFigs. 20.29 and 20.30). As tumors become more poorly differentiated, they tend to have less cytoplasm and increasingly basophilic cytoplasm, along with increasing degrees of nuclear atypia. The cytoplasm can have fatty change, clear cell change, or eosinophilic inclusions. Most HCCs grow in trabecular, solid, or pseudoacinar growth patterns (Fig. 20.15). There are many additional histologic findings that are discussed in more detail under specific variants.
Histochemical Stains and Immunostains
Most cases benefit from additional stains to help confirm the diagnosis. As discussed earlier, there are two distinctly different situations in which stains are used. The first situation is making a diagnosis on well-differentiated hepatic lesions; the second situation is to confirm hepatic differentiation in moderately to poorly differentiated carcinomas. It is best to make this distinction before you order your stains.
Some additional observations may be helpful in understanding the literature on immunostains and HCC. There is a large body of literature that pits one stain against another and has them duel it out to see who is number one for diagnosing HCC. Keep in mind these issues as you read this literature (or hear talks or read books that summarize this literature). First, a stain’s performance characteristics will depend on the grade of the tumor. For example, a general trend in the literature is that hepatocyte paraffin 1 (HepPar1) beats glypican 3 in well-differentiated tumors, whereas glypican 3 beats HepPar1 in poorly differentiated tumors. A second important observation is that other factors such as the presence or absence of cirrhosis and the underlying liver disease can also influence the overall performance of a stain. Thus, the published results from a given center can be strongly influenced by the overall mix of tumor grades in their study population as well as other histologic finding such as background fibrosis. A third important observation, which naturally follows the first two, is that in most cases, the first reports in the literature will have the highest sensitivities and specificities for a given marker; the sensitivities and specificities almost invariably drop off with additional experiences at other institutions. Finally, sometimes, a paper can be overly committed to the superiority of a single marker. In the end, however, most expert pathologists prefer to use a panel of stains in their daily practice and many published articles have drawn the same conclusion in the end.
A last note regarding staining specificity: Many immunostains that detect hepatic differentiation will also be positive in a wide range of non-HCCs. Although this is important to know, it is less of a problem than the literature sometimes makes it out to be because you also have a powerful ally: the H&E findings. As one example, a small proportion of adenocarcinomas from many different sites can be HepPar1-positive, but generally, this is only a small concern because adenocarcinomas typically do not look like HCCs on the H&E. Thus, as a practical matter, in most cases, this is not really a practical matter. For those cases that are truly problematic, for example, a biopsy with only a very small amount of poorly differentiated tumor, the best approach lies in doing a panel of stains. Hepatoid carcinomas from other organs that metastasize to noncirrhotic livers can be very challenging. CDXZ positivity can help identify these cases. Some will also be HepPar1-positive but arginase-1 negative.
Currently, immunostains in routine practice are performed only for diagnostic purposes. In the future, it is fully anticipated that immunostains will also be used to help select therapy as well as provide prognostic information.
CD34 STAIN. Immunostains for CD34 highlight the zone 1 sinusoids of the normal liver (eFig. 20.31), but in HCCs, there tends to be a strong diffuse sinusoidal staining pattern (eFig. 20.32). When the staining is strong and diffuse, this stain can be helpful. However, many times, HCCs will lack this staining pattern and CD34 is often not as useful as many of the other available stains. HCC with a macrotrabecular growth have a distinctive staining pattern where CD34 highlights the very thick trabeculae but does not otherwise stain the tumor (eFig. 20.33).
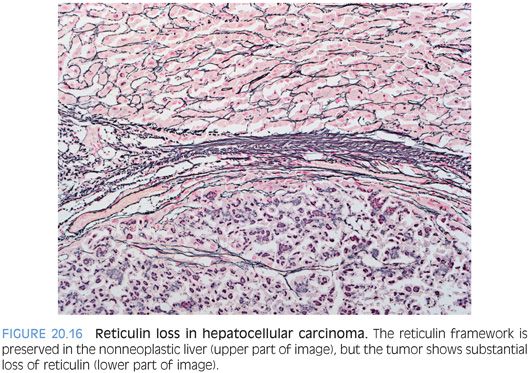
RETICULIN STAIN. The reticulin stain has been a true workhorse in the diagnosis of HCC. In the normal liver, the reticulin stain will highlight the hepatic trabecular architecture, with trabecula composed of single or double layers of hepatocytes. Sometimes, the trabeculae are even a bit thicker (three to four cells) in rapidly regenerating livers. In tumors, the hepatic trabeculae are even thicker and the reticulin stain brings this out. However, because many HCCs do not grow in trabecular growth pattern, “thickened trabecula” is probably not as useful of a diagnostic construct as “loss of reticulin.” In benign livers, all hepatocytes touch reticulin on at least one of their borders. With HCC, there is reduction in the amount of reticulin and there will be aggregates of hepatocytes that do not touch a reticulin fiber (Fig. 20.16). Regardless of which approach you prefer, remember that the reticulin findings should be more than focal and minimal to have diagnostic significance. Also of note, a small proportion of well-differentiated HCCs (less than 1%) will have a normal reticulin staining pattern on liver biopsy (see Fig. 20.7).19 The diagnosis in these cases has to be made by other cytologic features, including atypia and proliferative rates. An additional diagnostic pitfall is fatty tumors because benign livers with macrovesicular steatosis can have focal and patchy reticulin loss that mimics HCC.20