Bile duct hamartomas can be single or multiple. Most are small (less than 10 mm), but occasional cases can be very large, up to 20 cm, with large dilated biliary cysts admixed with areas of more typical hamartomas. The large cysts often appear to have risen out of the smaller cysts and all should lack ovarian type stroma. This rare entity has been referred to as giant cystic bile duct hamartoma.1
Immunostains
Immunostains are not needed to make the diagnosis, but hamartomas stain similar to normal bile ducts. They do have very low to absent Ki-67 labeling and this can be very helpful in some cases if you are worried about malignancy.
BILE DUCT ADENOMA
Definition
Bile duct adenoma is a benign aggregate of small tubular bile duct–like structures without lumens or bile, growing in variably fibrous stroma. A synonym is peribiliary gland hamartoma.
Demographics and Risk Factors
Bile duct adenomas also appear to be acquired lesions and are more commonly seen in cirrhotic livers than in noncirrhotic livers. Based on immunohistochemistry findings, they may arise from peribiliary glands and thus can also be called peribiliary gland hamartomas.2
There is some debate over whether these benign biliary lesions are neoplastic or reactive. They express proteins more commonly seen in peribiliary glands than bile ducts and have some features of pyloric gland metaplasia.2,3 Nonetheless, the overall currently available information does not clarify if they are neoplasms or hamartomas but does somewhat favor that they are at least acquired lesions and thus unlikely to be hamartomas in the literal sense of the word.
Histologic Findings
Bile duct adenomas are usually small, single, and subcapsular. Most are less than 1 cm in diameter, but occasional cases can measure several centimeters. In about 10% of cases, there can be multiple lesions. The adenomas are composed of small tubular structures that, in contrast to von Meyenburg complexes, lack lumens, do not interanastomose, and do not contain bile (Fig. 21.2, eFigs. 21.2 to 21.5). Cytologically, the cells should have consistent nuclear size, smooth round nuclei, and inconspicuous nucleoli. Rarely, the epithelial cells can also be oncocytic.4 Glandular complexity and luminal necrotic debris are not found. Bile duct adenomas are typically embedded in a fibrous stroma. Their appearance can vary from that of plump, small, round glands embedded in scant and loose fibrous stroma to that of atrophic-appearing glands and tubular structures embedded in dense fibrosis. Bile duct adenomas tend to be well circumscribed, but infiltrative borders with entrapped hepatocytes can occasionally be seen. The lesion often has mild lymphocytic inflammation. The epithelial cells of the lesion have a cytokeratin profile that is typical for biliary type cells. The Ki-67 rate should be low or absent, and mitotic figures are generally absent and never atypical. Rare cases of malignant degeneration have been reported, but their overall malignant potential is close to none.
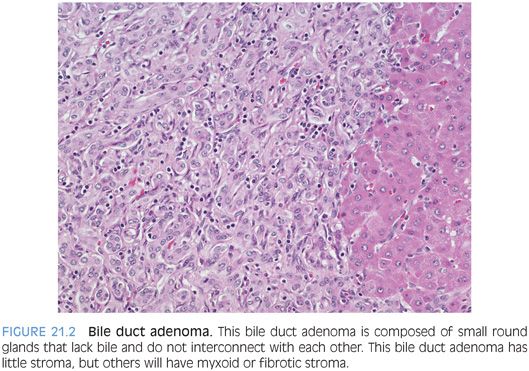
Sometimes, these tumors can be difficult to separate from a well-differentiated cholangiocarcinoma, especially on needle biopsy. These features would all favor cholangiocarcinoma: prominent nucleoli, significant variation in nuclear size, glandular complexity, mitotic figures, or luminal necrosis. In borderline cases, a firm diagnosis is sometimes impossible on needle biopsy and the best diagnosis is that of an atypical bile duct lesion followed with the differential. Bile duct adenomas stain like normal bile duct cells, and there are no routinely available immunostains that are specific for bile duct adenomas.
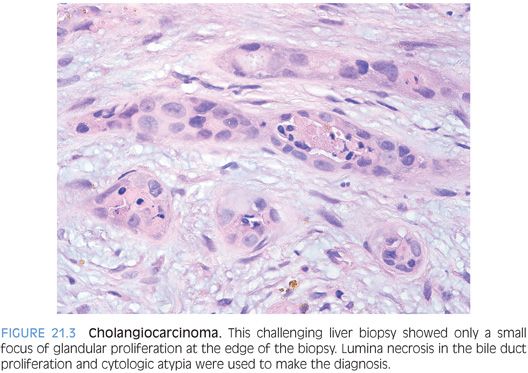
CHALLENGING BILE DUCT LESIONS. Many times, biopsies for a mass lesion will show an aggregate of bile duct–type structures and the differential is between a benign lesion and a well-differentiated cholangiocarcinoma. Benign lesions in the differential include ductular proliferation in response to a nearby, nonsampled mass lesion as well as a bile duct hamartoma or adenoma. Findings that can help sort these cases out include architectural features, cytologic findings, and proliferative rate. None is absolutely perfect in isolation and the features work best when considered together. A ductular proliferation that remains located at the periphery of fibrous bands would favor a benign reactive process. Luminal necrotic debris is a worrisome finding that would favor malignancy (Fig. 21.3). Another architectural finding that favors malignancy is that of “incomplete glands,” where a gland does not appear to be lined by epithelial cells around its entire circumference (Fig. 21.4). Cytologic atypia can be very helpful when present, but remember that some cholangiocarcinomas are very well differentiated. A Ki-67 immunostain typically shows increased proliferation in cholangiocarcinomas but minimal or absent staining in an adenoma or hamartoma. A ductular reaction can have a high proliferative rate also but typically only in cases of recent substantial parenchymal injury and/or collapse. In this situation, the ductular reaction can be more diffuse but still tends to retain a lobular pattern at low power. The proliferating ductules are often iron-positive. In addition, residual portal tracts and small islands of hepatocytes are common in areas of parenchymal collapse.

CLEAR CELL BILE DUCT ADENOMA
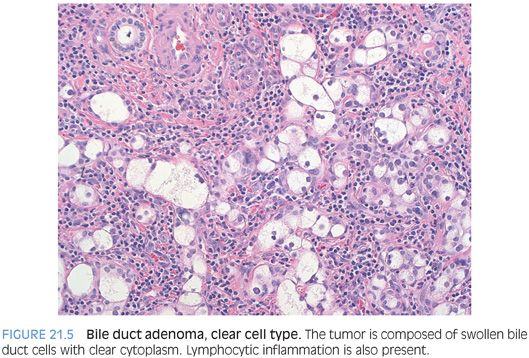
A rare but distinctive subtype is clear cell bile duct adenoma.5 The neoplastic cells have moderate amounts of cytoplasm, with diffuse clear cell change (Fig. 21.5). The cells grow in small nests and cords with round to ovoid nuclei that show little pleomorphism. They have a low proliferative rate by Ki-67 and otherwise stain-like typical adenomas. The borders can be less well defined than a typical adenoma, with extension into the adjacent hepatic parenchyma. They often have mild chronic inflammation (see Fig. 21.5, eFig. 21.6). The differential includes clear cell cholangiocarcinoma and metastatic clear cell carcinomas. The p53 and cytokeratin immunostains do not distinguish a clear cell bile duct adenoma from a clear cell cholangiocarcinoma. Instead, use the cytologic features (lack of atypia), lack of necrosis, and low proliferative rates to separate these two entities. Metastatic clear cell carcinomas have to be excluded by clinical and immunostain findings because the histologic findings can show overlap.
BILIARY ADENOFIBROMA
Definition
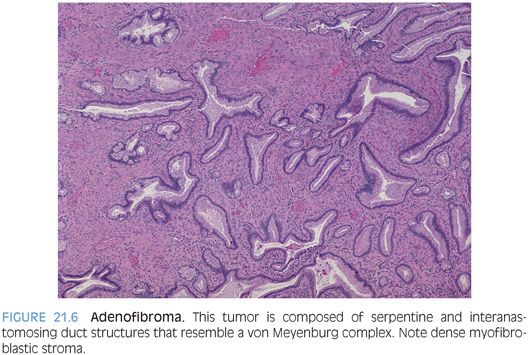
Biliary adenofibroma is a biliary tumor composed of tubulocystic structures that are dilated and embedded in a fibrotic stroma. This tumor is rare and has only been described relatively recently.6 However, it is probably a bit more common because many cases were most likely previously classified as von Meyenburg complexes or bile duct adenomas. They range in size from 5 to 20 cm.7 The biliary structures in adenofibromas tend to be larger than in adenomas or von Meyenburg complexes and can be filled with blood or proteinaceous material and sometimes bile. The glandular structures can be serpentine and interanastomosing in some areas, closely resembling classic von Meyenburg complexes (to which they may be related). Although data is limited, to date, mucin production has not been reported. These tumors are embedded in a dense fibrous stroma (Fig. 21.6) and often have entrapped benign hepatocytes. The epithelial cells stain for CK7 and CK19, whereas the stromal cells are myoblastic in nature and strain strongly for both vimentin and smooth muscle actin.
SIMPLE CYSTS: BILIARY, MESOTHELIAL, AND FOREGUT
Definition
Simple cyst is a solitary cyst lined by simple cuboidal epithelium and lacking ovarian stroma. Simple biliary cysts are sometimes called solitary bile duct cysts. A simple biliary cyst is only rarely biopsied but can sometimes be removed by a wedge biopsy (eFig. 21.7). Simple cysts have a strong female predominance (8:1), and the average age is in the sixth decade. They are asymptomatic in half of the cases. Grossly, they are usually a single cyst but occasionally can be an aggregate of several smaller cysts. The cysts are lined by simple biliary type epithelium that lies on a dense layer of fibrous tissue. The epithelium may undergo metaplasia (intestinal, pyloric, squamous) and occasionally dysplasia. In most cases, the epithelium is extensively sloughed off during the processing and you will have to hunt to find the small remaining bits of epithelium. If the cyst is in the hilum or near a large intrahepatic portal tract, it can arise from the peribiliary glands and is called a peribiliary gland cyst.
The wall may contain small aggregates of bile ducts or peribiliary type glands. If the cyst has ruptured, the wall can have numerous pigment-laden macrophages that give a cellular appearance. No ovarian stroma should be seen. The differential also includes mesothelial cysts. Mesothelial cysts are generally small and always subcapsular. They are lined by mesothelial cells instead of biliary type cells, a distinction that is often difficult without the use of immunostains. Sometimes, the mesothelial cells can have a hobnail appearance. The surrounding fibrous stroma can have entrapped portal tracts and single and small aggregates of hepatocytes. Mesothelial cells are positive for calretinin, WT1, CAM5.2, cytokeratin AE1/3, epithelial membrane antigen (EMA) (about 15% of cases), desmin, and vimentin. Mesothelial cysts are most commonly seen as small intraoperative biopsies when doing other abdominal surgeries.
Finally, if the epithelium lining the cyst is columnar and has cilia, then the diagnosis should be a ciliated hepatic foregut cyst. Smooth muscle bundles are often seen in the walls of the cyst. Ciliated hepatic foregut cysts are rare but equal in frequency in men and women and average 4 cm at the time of surgery—although they can occasionally reach sizes greater than 10 cm. They are unilocular in 90% of cases.8 Ciliated foregut cysts are less common than mesothelial cysts, but they also tend to be subcapsular and so are most likely to be encountered as intraoperative biopsies when a surgeon is doing other abdominal surgeries. Another common presentation is as an incidental finding on imaging studies.
MUCINOUS CYSTIC NEOPLASM
Definition
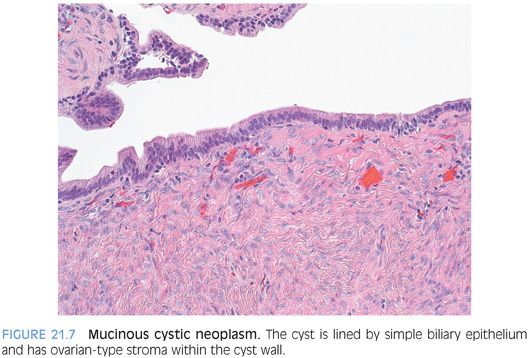
Mucinous cystic neoplasm is a cyst lined with biliary type epithelium with ovarian type stroma in the cyst wall.
Demographics and Risk Factors
Mucinous cystic neoplasms used to be called biliary cystadenomas, hepatobiliary cystadenomas, or cystadenocarcinomas (when malignant). The 2010 edition of the WHO Classification of Tumours of the Digestive System classifies mucinous cystic neoplasms into three subtypes: low- or intermediate-grade neoplasia, high-grade neoplasia, or mucinous cystic neoplasm with invasive carcinoma. They are similar to mucinous cystic neoplasms of the pancreas. There is a strong female predominance (approximately 20:1), and most occur in the fourth and fifth decade of life.
Histologic Findings
Mucinous cystic neoplasms are solitary and multilocular. The fluid content is generally thin and clear but can be more mucinous, bloody, or purulent if the cyst has become infected. The cyst should not communicate with the bile ducts. If it does, then the case is much more likely to be an intraductal papillary neoplasm with marked cystic change. In fact, using the combined features of ovarian stroma and lack of communication with bile ducts will separate mucinous cystic neoplasms from intraductal papillary neoplasms in almost all cases.9
The cysts are lined by epithelial cells that are classically columnar with basally oriented nuclei and apical-oriented mucin but can also be cuboidal and even flattened. The epithelium expresses a range of cytokeratins including CK7, CK19, CK8, and CK18. CK20 will be negative, except in areas of dysplasia.9 Synaptophysin or chromogranin stains will highlight scattered endocrine cells in the epithelial lining. Intestinal metaplasia is common, and pyloric gland metaplasia and squamous metaplasia can also be seen. Special stains, such as mucicarmine or Alcian blue, will highlight the mucinous nature of the epithelium, but choose areas with columnar cells to do your stain. Not all mucinous cystic neoplasms of the liver will have demonstrable mucin, especially if your section or biopsy has mostly cuboidal or flattened epithelium. However, the diagnosis can still be made without the mucinous features as long as the ovarian type stroma is present (Fig. 21.7). Furthermore, ovarian type stroma is required to make the diagnosis. Ovarian type stroma is composed of spindle-shaped cells that are positive for estrogen receptor, progesterone receptor, and α-inhibin by immunostaining.10 Vimentin, actin, and desmin stains also are positive.