Barrett’s esophagus is an important step in the pathway to esophageal adenocarcinoma. Since most patients with Barrett’s esophagus are undiagnosed and patients present with advanced adenocarcinoma de novo, prognosis for this disease remains poor. To identify those people with Barrett’s esophagus who are at particular risk many new technologies are being developed. In association with these advances in risk stratification, progress is being made in the endoscopic treatment of Barrett’s. Chemoprevention is also an area of interest and trials are underway.
The tools available for the investigation and management of Barrett’s esophagus (BE) have advanced significantly over the past 5 years. This article summaries the recent advances in BE, including ways of classifying BE and stratifying the risk of progression to esophageal adenocarcinoma (AC). The ways in which advances in endoscopic modalities are impacting diagnosis and treatment are also discussed.
Definition
BE is defined as a metaplastic replacement of the normal squamous epithelium lining of the distal esophagus by columnar epithelium. Three histopathologic subtypes of esophageal columnar metaplasia have been elucidated. The first two exhibit a gastric phenotype and may be either fundic or cardiac-junctional (nonsecretory) in nature. The third phenotype is intestinal and defined by the presence of goblet cells. Most BE is comprised of a combination of metaplastic subtypes with intestinal metaplasia (IM) being the predominant phenotype in adults. There does not seem to be a relationship between the metaplastic phenotype and the position within the BE segment. The American College of Gastroenterology guidelines, last updated in 2008, specify that the term BE should be restricted to columnar-lined epithelium containing IM because this is the subtype with the greatest risk for neoplastic progression.
Esophageal AC develops from BE by a metaplasia-dysplasia-adenocarcinoma sequence. In most AC cases there is no prior diagnosis of BE; however, in patients with known BE the risk of developing AC is approximately 0.5% each year.
Epidemiology and risk factors
Gastroesophageal reflux disease (GERD) is the major risk factor associated with development of BE. Approximately 44% of adults in the United States have GERD symptoms at least once per month. BE is found in 3% of patients undergoing endoscopy for reflux symptoms in the United States and 1% of all those undergoing endoscopy. A comparison of patients with GERD symptoms indicated that those with BE had a longer duration of symptoms compared with patients with esophagitis; those who had GERD symptoms for more than 13 years were shown to have a 1.5-fold increased risk of having BE. In keeping with the GERD symptoms in BE patients most have a hiatus hernia and the latter may be longer and wider than in patients undergoing endoscopy who have no evidence of BE.
The incidence of AC rose almost sixfold between 1975 and 2001 in the United States. The incidence of BE may be increasing in a similar manner. One study shows a 62% increased incidence of BE between 1997 and 2002 (from 14.3 per 100,000 person-years to 23.1 per 100,000 person-years) and this increase was shown to be independent of the number of gastroscopies. Some of the perceived increase in incidence of BE may be caused by increasing awareness, and the acceptance that short segments of BE (<3 cm) exist, and not all studies confirm the increasing incidence. Both BE and AC occur approximately three times more commonly in men than women. The prevalence of BE increases with age and plateaus in the seventh decade. It is suspected, however, that BE may develop 20 years before diagnosis.
There have been conflicting reports recently on the relationship between adiposity and the risk of BE and AC. One such study observed that measures of central adiposity in a group of patients with a new diagnosis of BE were significant risk factors for BE, even after accounting for potential confounding factors (eg, age, gender, alcohol and cigarette use). Two meta-analyses of the relationship between body mass index and the risk of BE have, however, failed to show evidence of an association above what is expected from GERD alone. In those with reflux symptoms, body mass index cannot be used to stratify the risk of BE. In a large public health survey in Norway a dose-response relationship was found between increasing body mass index and reflux symptoms. In addition to the possibility that increased body mass index can lead to increased reflux symptoms because of anatomic disruption of the gastroesophageal junction, it has been hypothesized that adipocytes within visceral fat may contribute to the development of BE by the production of such factors as leptin, adiponectin, or cytokines. Leptin is a candidate of particular interest because its levels are related to the volume of fat cells present and it has been shown to have mitogenic and angiogenic activities. Leptin has been implicated in cancer of the prostate, breast, and gastrointestinal tract.
A diet high in fruit and vegetables has been associated with a decreased risk of BE, which has been attributed to the higher levels of antioxidants in these products. Vitamin C may provide additional benefit by reducing nitrosating species, which might otherwise form carcinogenic N -nitroso compounds, which have been implicated in the pathogenesis of BE. Although the body derives these nitrosating species from dietary nitrate, which is principally ingested from fruits and vegetables, a healthy diet seems to be protective overall. Diets high in animal fats may promote the development of BE, perhaps through alteration in the bile-acid content of refluxate. Smoking and alcohol do not seem greatly associated with BE, unlike their strong association with esophageal squamous cell carcinoma.
Epidemiology and risk factors
Gastroesophageal reflux disease (GERD) is the major risk factor associated with development of BE. Approximately 44% of adults in the United States have GERD symptoms at least once per month. BE is found in 3% of patients undergoing endoscopy for reflux symptoms in the United States and 1% of all those undergoing endoscopy. A comparison of patients with GERD symptoms indicated that those with BE had a longer duration of symptoms compared with patients with esophagitis; those who had GERD symptoms for more than 13 years were shown to have a 1.5-fold increased risk of having BE. In keeping with the GERD symptoms in BE patients most have a hiatus hernia and the latter may be longer and wider than in patients undergoing endoscopy who have no evidence of BE.
The incidence of AC rose almost sixfold between 1975 and 2001 in the United States. The incidence of BE may be increasing in a similar manner. One study shows a 62% increased incidence of BE between 1997 and 2002 (from 14.3 per 100,000 person-years to 23.1 per 100,000 person-years) and this increase was shown to be independent of the number of gastroscopies. Some of the perceived increase in incidence of BE may be caused by increasing awareness, and the acceptance that short segments of BE (<3 cm) exist, and not all studies confirm the increasing incidence. Both BE and AC occur approximately three times more commonly in men than women. The prevalence of BE increases with age and plateaus in the seventh decade. It is suspected, however, that BE may develop 20 years before diagnosis.
There have been conflicting reports recently on the relationship between adiposity and the risk of BE and AC. One such study observed that measures of central adiposity in a group of patients with a new diagnosis of BE were significant risk factors for BE, even after accounting for potential confounding factors (eg, age, gender, alcohol and cigarette use). Two meta-analyses of the relationship between body mass index and the risk of BE have, however, failed to show evidence of an association above what is expected from GERD alone. In those with reflux symptoms, body mass index cannot be used to stratify the risk of BE. In a large public health survey in Norway a dose-response relationship was found between increasing body mass index and reflux symptoms. In addition to the possibility that increased body mass index can lead to increased reflux symptoms because of anatomic disruption of the gastroesophageal junction, it has been hypothesized that adipocytes within visceral fat may contribute to the development of BE by the production of such factors as leptin, adiponectin, or cytokines. Leptin is a candidate of particular interest because its levels are related to the volume of fat cells present and it has been shown to have mitogenic and angiogenic activities. Leptin has been implicated in cancer of the prostate, breast, and gastrointestinal tract.
A diet high in fruit and vegetables has been associated with a decreased risk of BE, which has been attributed to the higher levels of antioxidants in these products. Vitamin C may provide additional benefit by reducing nitrosating species, which might otherwise form carcinogenic N -nitroso compounds, which have been implicated in the pathogenesis of BE. Although the body derives these nitrosating species from dietary nitrate, which is principally ingested from fruits and vegetables, a healthy diet seems to be protective overall. Diets high in animal fats may promote the development of BE, perhaps through alteration in the bile-acid content of refluxate. Smoking and alcohol do not seem greatly associated with BE, unlike their strong association with esophageal squamous cell carcinoma.
Screening
Heartburn symptoms have been established as a major risk factor for the development of AC (odds ratio, 7.7), a risk that increases with duration and severity of symptoms. This raises the question whether heartburn symptoms could be used, perhaps in combination with other known risk factors, such as age and gender, to inform a screening strategy for BE. The absolute risk of developing AC in those with reflux, however, remains small. This small risk must be weighed against the cost, psychologic burden, and small risk of perforation associated with endoscopic screening. As a result of the controversy surrounding screening, the international recommendations diverge. Screening is not currently recommended by the British Society of Gastroenterology; however, the recent American Society of Gastroenterology guidelines suggest screening endoscopy for those over the age of 50 who have chronic GERD symptoms.
Surveillance
In those who are known to have BE, the increased risk of AC means that surveillance is closer to meeting the Wilson-Junger criteria. Even so, one case of AC is likely to be detected each 200 patient-years of surveillance. This figure does not take into account the cases of high-grade dysplasia that can be found and treated and prevent AC formation. Asymptomatic AC that is found at surveillance is generally at an earlier stage and carries an improved prognosis, some of which may be accounted for by “lead-time bias,” which is difficult to quantify.
The American College of Gastroenterology suggests those with nondysplastic BE undergo gastroscopy every 3 years, although shorter time periods should be implemented if dysplasia is present. The British Society of Gastroenterology does not recommend surveillance for all, but suggests patients should be given an informed choice, and those who wish to undergo surveillance should (in the absence of dysplasia) have endoscopy every 2 years.
Psychologic burden
Before undertaking either a screening or surveillance program account must be taken of the resulting psychologic burden. BE surveillance patients do report that the procedure is a burden to them with “increased anxiety, depression, and distress” in the week before endoscopy. It must be remembered, however, that this group of patients actually report less depression and anxiety than those undergoing gastroscopies for investigation of symptoms and this is independent of the use of sedation. It is likely that patients who understand the indication for entry into a surveillance program, and who decide to take part, are those more able to adapt to tolerate the program.
Risk stratification
Classification systems of BE have been developed to stratify the risk of progressing to AC, and focus and optimize surveillance programs. Surveillance programs aim to detect cancers at a curable presymptomatic stage, because symptomatic presentation often occurs late and carries a poor prognosis (14% 5-year survival). Risk stratification can be performed on the basis of the endoscopic findings, the histopathologic findings, or the use of other biomarkers.
Endoscopic assessment and risk stratification
Length of Barrett’s Segment (White-Light Endoscopy)
In a multicenter retrospective observational study, the annual incidence of AC was significantly higher if the BE segment length was greater than 9 cm. A retrospective analysis found each centimeter increase in segment length equated with a 17% increase in the risk of developing high-grade dysplasia or AC. This association with segment length has not been universally found; a prospective cohort study of patients with BE showed no significant difference in AC risk between short and long segments (using 3 cm as the cut-off).
Measurement of BE segment length is not without its difficulties. Studies comparing the segment length in serial endoscopies have demonstrated variation of more than 4 cm in 10% of patients. This variability can be accounted for perhaps by peristalsis and respiration, the degree of air insufflation, lack of small gradations on standard endoscopes, and the presence of hiatus hernias.
The Prague or “CM” classification is an attempt to standardize the reporting of BE length. This specifies the maximum number of centimeters of columnar epithelium regardless of whether it is only a tongue or confluent (the M score), and the total number of centimeters of circumferential metaplasia from the gastroesophageal junction (the C score).
Confocal Fluorescence Laser Microscopy
White-light endoscopy provides a large field of view at a relatively poor resolution, despite the advances that have been made with the optical zoom function and higher pixel density. Confocal fluorescence laser microscopy aims to provide sharp resolution of a small area with visualization below the epithelial surface. This technique offers the promise of in vivo histology; however, the light penetration has been relatively poor thus far (in the region of 200 μm below the contact surface).
Confocal fluorescence laser microscopy can make use of autofluoresence of the mucosa, when excited by ultraviolet light of suitable wavelength. The wavelengths needed for autofluoresence do not, however, provide a good depth of resolution. This can be overcome with the use of exogenous fluorophores. Intravenous fluorescein has been used because it becomes deposited in the extracellular matrix of the lamina propria; because it is not miscible with mucin it does not penetrate into goblet cells, which have a dark appearance. Acriflavine is another exogenous fluorophore that labels acidic intracellular constituents. Topical application of acriflavine results in superficial epithelial staining, whereas goblet cells remain unstained. This technique has been successfully used in both colonoscopy and gastroscopy where it clearly defines the mucosal type and pit pattern. It has been used to demonstrate the presence of goblet cells in BE.
Chromoendoscopy
Various chromophores have been used to improve detection of IM at endoscopy, including methylene blue, Lugol’s iodine, acetic acid, and indigo carmine. All of these techniques are reliant on the dye-spraying technique, removal of mucus, and thoroughness of washing after staining, which makes the staining rather subjective and time-consuming.
Lugol’s iodine binds to polysaccharides, in particular glycogen in squamous epithelium, which it stains brown. Metaplastic columnar epithelium stains poorly, but is highlighted by the absence of stain. Methylene blue, in contrast, is actively taken up by areas of IM, staining them blue, whereas it is not taken up by native squamous epithelium. Acetic acid breaks up the surface mucous and causes reversible deacetylation of nuclear and cytoplasmic proteins. The mucosa becomes white and edematous, emphasizing the pit pattern. This technique has been used to emphasize persistent islands of BE following endoscopic ablation. Methylene blue is a stain that is selective for columnar metaplasia and the staining in high-grade dysplasia and cancer is more heterogeneous with an overall lower intensity.
Chromoendoscopy can be assisted by the use of high magnification, which has allowed identification of pit patterns representative of high-grade dysplasia ( Table 1 ). In a series of 80 patients with suspected BE, who were examined after spraying with indigo carmine, this mucosal pit pattern classification system had a sensitivity of 97% and a specificity of 76% giving a positive predictive value of 92% for the presence of IM. All patients with an irregular or distorted mucosal pattern were found to have high-grade dysplasia.
| Pattern | Classification | Description |
|---|---|---|
| Mucosal pattern | Ridge/villous | Longitudinal darker and lighter ridges distributed in a uniform manner |
| Circular | Uniform circular pattern | |
| Irregular/distorted | Nonuniform, irregular, and distorted pattern | |
| Vascular pattern | Normal vascularity | Thin vessels with a uniform branching pattern |
| Abnormal vascularity | Dilated, corkscrew vessels with increased vascularity and abnormal, nonuniform branching pattern |
Autofluorescence
Autofluoresence does not require the administration of an exogenous fluorophore, but rather relies on the natural fluorescent characteristics of tissue, such as elements of connective tissue (eg, collagen, elastin); enzymes (eg, NADH, FAD); amino acids (eg, tryptophan, tyrosine, phenylalanine); or hemoglobin break-down products (eg, porphyrin). Attempts have been made, with mixed success, to use autofluoresence to demonstrate areas of high-grade dysplasia. An initial randomized study to compare autofluoresence with traditional white-light endoscopy showed no improvement in the detection of high-grade dysplasia.
Narrow Band Imaging
Narrow band imaging filters out the longer wavelengths of light, improving the clarity of the image and emphasizing the mucosal vasculature. This technique eliminates the need for application of dyes and tends to be used in association with magnification, which means that inspection of only small areas of mucosa are possible at a time. Mucus can interfere with picture quality and may require endoscopic flushing with water or N -acetylcysteine or simethicone.
Several small studies have investigated the ability of narrow band imaging to predict accurately the histologic findings. A classification of the mucosal-vascular patterns seen with narrow band imaging has been adapted from a chromoendoscopy classification system (see Table 1 ). A study of 51 BE patients was able to identify 95% of cases of IM without dysplasia, by the presence of “a characteristic ridge/villous pattern with a uniform vascular distribution.” All seven cases of high-grade dysplasia had “an irregular/distorted pattern with tortuous, abnormally branching, non-uniform vascular pattern.” This technique and classification was unable to detect low-grade dysplasia.
Combination Approach
The very high false-positive rate (40%) of autofluorescence, despite its high sensitivity (100%), has led to suggestions that it may be particularly useful as an initial screening method. The wide field of view allows for detection of suspicious areas, which can then be inspected using another endoscopic modality to reduce the false-positive rate.
To avoid the need for sequential endoscopies a “trimodal” system has been developed that combines the ability to perform high-resolution white-light endoscopy, narrow band imaging, and autofluoresence by a single endoscope ( Fig. 1 ). This trimodal technique was tested in a multicenter study against the gold standard of random quadrantic biopsies for the identification of high-grade dysplasia and carcinoma in situ within BE and was shown to increase detection of early neoplasia.
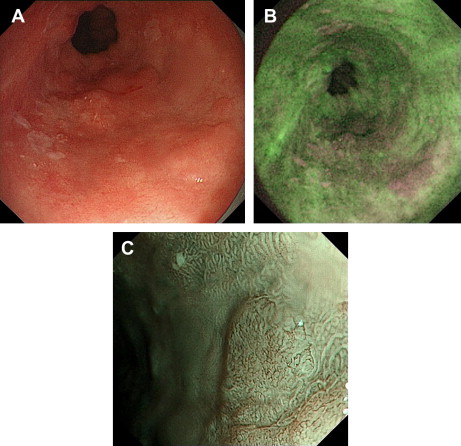
In a prospective multitertiary referral center study of 84 participants with BE, the addition of autofluoresence to high-resolution endoscopy (which was regarded as the current gold standard) increased detection of early neoplasia, at the expense of a false-positive rate of 81%. This false-positive rate could be reduced to 26% with the sequential use of narrow band imaging. An international prospective multicenter trial of trimodal imaging showed it to improve detection of dysplasia in BE from 48% to 89%. Another study from the same group, however, examined the optimal endoscopic tool to evaluate the mucosa and vasculature in BE from still images, obtained by chromoendoscopy and narrow band imaging. Although expert endoscopists expressed a preference for images obtained with chromoendoscopy and narrow band imaging rather than white-light endoscopy, neither of these newer techniques could be shown to improve recognition of dysplasia above high-resolution white-light endoscopy.
Transnasal Endoscopy
Transnasal endoscopy with an outer diameter of less than 6 mm has been developed to improve tolerability and remove the need for sedation in patients undergoing surveillance. This has the potential to be used as a screening tool. Several types of transnasal endoscope are available with four-directional or two-directional angulation of the tip. When biopsies are taken, the availability of four-way angulation has been shown to decrease examination time while not significantly altering tolerability. Another study has, however, demonstrated improved tolerability and success with the use of the finer bidirectional endoscope.
In a small study to examine the feasibility and accuracy of the use of the transnasal route in BE, it was found that despite the smaller size of biopsy, obtained with pediatric forceps by the nasal endoscope, there was no significant difference between the histologic diagnosis of IM or dysplasia when compared with traditional oral endoscopy.
Optical Techniques in Development
Elastic scattering spectroscopy aims to remove the problem of interobserver variability in the interpretation of histology from biopsies or pit patterns obtained with other endoscopic imaging modalities. It relies on the light scattering index of cellular components, such as nuclear structures and membranes. The spectral signal obtained, in less than a second, can be compared with spectra from normal and abnormal tissues. At present this probe samples only 1 mm 2 of mucosa, leaving the residual problem of sampling error. Preliminary data suggest a sensitivity of 71% for detecting high-grade dysplasia or cancer and a negative predictive value of 96%.
Optical coherence tomography, which uses light near the infrared spectrum, has been adapted for use in an endoscope and can provide cross-sectional images of the mucosa with micrometer resolution to a depth of a millimeter. This allows the identification of a layered structure, glandular structures, or the presence of pits and crypts and in this way can be used to identify IM with a sensitivity of 85%, although it is poor at differentiating between IM and gastric metaplasia.
Raman (inelastic) spectroscopy also uses light from the near infrared range. The interaction of this light with protons in tissue results in inelastic scattering of the light in a fashion dependent on its molecular structure. This has been shown to be a potentially useful tool in detecting gastric dysplasia.
Histologic assessment and risk stratification
The traditional method of identifying those of particular risk of progression to AC has been to examine for high-grade dysplasia. In addition to biopsies from areas that appear abnormal, such as ulcers or areas of nodularity, the Seattle protocol suggests random quadrantic biopsies be taken approximately every 2 cm. This protocol has arisen because areas of dysplasia may appear identical macroscopically to adjacent nondysplastic areas. The endoscopic techniques being developed aim to allow specific targeting of biopsies and replace the traditional “hit and miss” method.
Low-grade dysplasia is generally perceived to be a poor biomarker because it is associated with a low rate of progression to AC. This risk may, however, be an underestimation; an expert histopathologic review of all cases of low-grade dysplasia in a multicenter study revealed that 77% were restaged to having no dysplasia at all and had negligible risk of progression in the 42-month follow-up period. In contrast, those in whom low-grade dysplasia was confirmed had a risk of progression to high-grade dysplasia or AC of 50% in the same time period. The rate of progression of high-grade dysplasia to AC varies from study to study and this may represent differences in the pathologic definition of dysplasia. Although high-grade dysplasia is the only biomarker for progression to AC currently in clinical use, it is far from ideal because of the low rate of progression to AC (one study reported a rate of 16% over the 7.3-year follow-up period). The Vienna classification was produced in 2000 to unify the pathologists’ description of the degree of dysplasia ( Table 2 ).
| Category | Classification |
|---|---|
| 1 | Negative for neoplasia/dysplasia |
| 2 | Indefinite for neoplasia/dysplasia |
| 3 | Noninvasive low-grade neoplasia |
| 4 | Noninvasive high-grade neoplasia |
| 5 | Invasive neoplasia |
Biomarkers and risk stratification
The Early Detection Research Network ( http://edrn.nci.nih.gov/ ) aims to develop and test putative biomarkers for the early detection of cancer. A summary of the progression of BE biomarkers through the Early Detection Research Network stages is shown in Fig. 2 . To transfer the use of molecular markers to the clinic they must be validated and applicable to routine clinical use. Ideally, the biomarker should not depend on the assessment of multiple random biopsies. Brushings are quicker to take than random biopsies, and allow sampling of a greater area; there are encouraging data applying immunomarkers, such as cyclin A and automated fluorescence in situ hybridization system, to these samples.
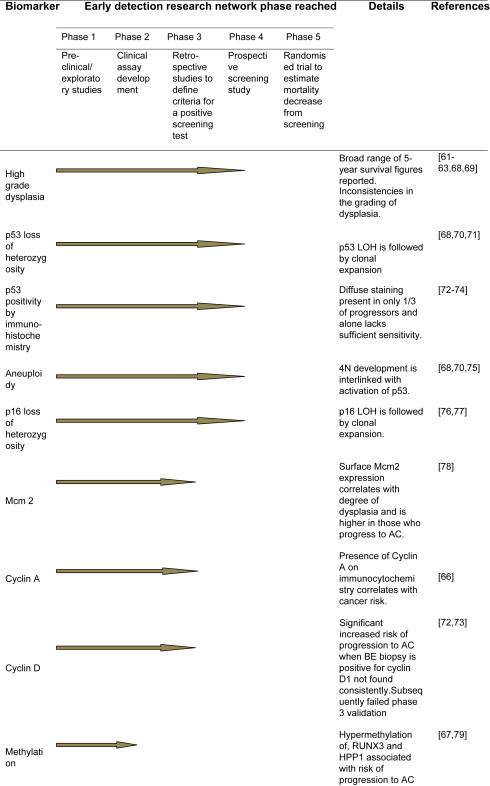
Because of the heterogeneity of the Barrett’s epithelium individual biomarkers lack sensitivity and specificity to predict progression to AC. The relative risk of individual biomarkers that are associated with progression to AC after 10-years of follow-up include 17p loss of heterozygosity (relative risk, 10.6); 9p loss of heterozygosity (relative risk, 2.3); TP53 mutation (relative risk, 7.3); tetraploidy (relative risk, 8.8); and aneuploidy (relative risk, 8.5). The combination of 17p loss of heterozygosity, 9p loss of heterozygosity, and DNA-content abnormality has been shown to predict the 10-year AC risk better than any single biomarker alone (relative risk, 38.7). In those with no abnormalities of their biomarkers at baseline, 12% developed AC over 10 years. In contrast, those with the combination of 17p loss of heterozygosity, 9p loss of heterozygosity, and DNA-content abnormality had a cumulative incidence of AC of 79% over the same period. The use of nonsteroidal anti-inflammatory drug prophylaxis had a protective effect and there was decreased progression to AC in nonsteroidal anti-inflammatory drug users, particularly those with multiple high-risk molecular changes.
Epigenetic changes, in the form of hypomethylation and hypermethylation and alteration to histone complexes, have also been implicated in the progression of BE to AC. During the transition from squamous epithelium to BE, hypermethylation of A-kinase anchoring protein 12 (AKAP12) and tachykinin1 (TAC1) occurs. The hypermethylation of these two genes increases along the pathway to dysplasia and cancer and they have been suggested as putative biomarkers. Methylation of the tumor suppressor gene CDKN2A can lead to its inactivation and is associated with a relative risk of 2.1 of developing AC during 10-years of follow-up.
Serum biomarkers allow the stratification of risk in an out-patient setting with minimal invasiveness. Antibodies to the p53 gene have been identified in the serum of those with dysplastic BE and AC. AC is often associated with hypermethylation of the APC gene and the levels of hypermethylated APC gene DNA in the plasma have been suggested as a possible plasma marker in AC.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




