CHAPTER 44 Barrett’s Esophagus
Barrett’s esophagus is the condition in which an abnormal columnar epithelium that is predisposed to malignancy replaces the stratified squamous epithelium that normally lines the distal esophagus.1 The condition is named for Norman Barrett, an Australian surgeon who drew attention to the columnar-lined esophagus in 1950.2 Barrett’s esophagus is a consequence of chronic gastroesophageal reflux disease (GERD), which damages the esophageal squamous epithelium and causes it to heal through a metaplastic process in which columnar cells replace reflux-damaged squamous cells. The columnar-lined esophagus causes no symptoms, and the condition has clinical importance only because it is a risk factor for esophageal adenocarcinoma, a tumor whose frequency has increased more than six-fold over the past several decades.3
DIAGNOSIS
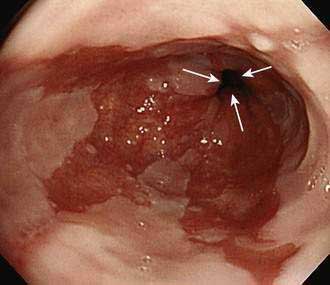
Barrett’s esophagus is diagnosed by endoscopic examination, and two criteria must be fulfilled. First, the endoscopist must ascertain that columnar-appearing epithelium lines the distal esophagus. Second, biopsy specimens of that columnar-appearing epithelium must show evidence of metaplasia, which is a change from one adult cell type to another. To ascertain that columnar-appearing epithelium lines the distal esophagus, the endoscopist first must locate the gastroesophageal junction (GEJ, which is recognized as the most proximal extent of the gastric folds), and then determine that columnar-appearing epithelium extends above the GEJ into the esophagus (Fig. 44-1). Endoscopically, columnar epithelium has a reddish color and velvet-like texture that can be distinguished readily from normal esophageal squamous epithelium, which is pale and glossy. There is disagreement among experts regarding the histologic type of epithelium required to confirm that there is evidence of metaplasia in the esophagus.4 Virtually all would agree that the finding of an intestinal-type epithelium with goblet cells (which has been called intestinal metaplasia, specialized intestinal metaplasia, or specialized columnar epithelium) is clear evidence of metaplasia. Most published studies on Barrett’s esophagus have used intestinal metaplasia as a requisite diagnostic criterion. However, some authorities argue that gastric cardiac-type epithelium, which is composed almost exclusively of mucus-secreting cells, also is metaplastic, has malignant predisposition, and can be considered diagnostic of Barrett’s esophagus.5,6 This debate remains unresolved.
Barrett’s esophagus can be further categorized as long-segment (when the metaplastic epithelium extends at least 3 cm above the GEJ) or short-segment (when <3 cm of metaplastic epithelium lines the esophagus).7 Another more recently proposed system for categorizing Barrett’s esophagus, the Prague C and M criteria, identifies the circumferential (C) and the maximum extent (M) of Barrett’s metaplasia.8 Data suggest that the cancer risk in Barrett’s esophagus may vary with the extent of the metaplastic lining. However, the clinical value of the proposed classification systems has not been established and presently patients with any extent of Barrett’s metaplasia are managed similarly.
EPIDEMIOLOGY
Barrett’s esophagus typically is discovered during endoscopic examinations performed for the evaluation of GERD symptoms in middle-aged and older adults.9 The average age at the time of diagnosis is approximately 55 years. The condition is rare in children younger than age 10 and virtually nonexistent in children younger than age 5.10 White men predominate in most series and, for unknown reasons, Barrett’s esophagus is uncommon in black and Asian populations. Among adult patients who have endoscopic examinations because of GERD symptoms, long-segment Barrett’s esophagus is found in 3% to 5%, whereas 10% to 20% have short-segment Barrett’s esophagus.1 In the general adult population of Western countries, the prevalence of Barrett’s esophagus (predominantly short-segment) is between 1.6% and 6.8%.11,12
Published estimates on the annual incidence of cancer in patients with long-segment Barrett’s esophagus have ranged from 0.2% to 2.9%, but it has been shown that many of those estimates were based on older, small studies that suffered from publication bias. Modern, larger studies, which are less susceptible to such bias, suggest that the risk of cancer in the general population of patients with Barrett’s esophagus is approximately 0.5% per year.13
The epidemiology of esophageal adenocarcinoma is similar to that of Barrett’s esophagus. GERD is strongly associated with both conditions and, like Barrett’s esophagus, esophageal adenocarcinoma affects white men predominantly.9,14 Obesity, especially with central adiposity, predisposes to both Barrett’s esophagus and esophageal adenocarcinoma,14,15 and the dramatic rise in the frequency of obesity in the United States has paralleled a similar rise in the prevalence of Barrett’s cancer. The mechanisms underlying these associations with obesity are not clear, but may relate to the fact that central adiposity predisposes to GERD, perhaps by increasing intra-abdominal pressure16 (see also Chapters 43 and 46). Obesity also is associated with elevated serum levels of pro-proliferative hormones such as insulin-like growth factor I (IGF I) and leptin, and with decreased levels of the antiproliferative hormone adiponectin, factors that may contribute to carcinogenesis in Barrett’s esophagus.
It has been proposed that the declining frequency of infection with Helicobacter pylori in Western populations also may be contributing to the rising frequency of esophageal adenocarcinoma (see Chapter 46). A number of studies have suggested that H. pylori infection may protect against the development and neoplastic progression of Barrett’s esophagus, perhaps because, in a subset of patients, this infection may prevent GERD by decreasing gastric acid secretion.17 Other factors that appear to protect against the development of esophageal adenocarcinoma include the use of aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs),18,19 and the consumption of a diet high in fruits and vegetables.18 Although cigarette smoking and alcohol consumption are very strong risk factors for squamous cell carcinoma of the esophagus, cigarette smoking only modestly increases the risk for esophageal adenocarcinoma and alcohol does not appear to affect that risk at all.18
PATHOGENESIS
Patients with long-segment Barrett’s esophagus often have severe GERD (see Chapter 43). Table 44-1 lists some physiologic abnormalities that have been reported in Barrett’s patients, and suggests how those abnormalities might contribute to GERD severity. Individual patients may exhibit any, all, or none of those abnormalities, and their prevalence in Barrett’s esophagus is disputed. For example, some investigators have described normal gastric acid secretion in patients with long-segment Barrett’s esophagus.20 In addition, many patients with short-segment Barrett’s esophagus have no GERD symptoms and no endoscopic signs of esophagitis. Indeed, one large study has suggested that short-segment Barrett’s esophagus may affect approximately 5% of adults, irrespective of the presence of GERD symptoms.11 Studies have shown that even in healthy volunteers, the very distal esophagus can be exposed to acid for more than 10% of the day.21 Such acid exposure can damage the esophagus directly and indirectly when nitrite (generated from dietary nitrate) reacts with acid to produce nitric oxide. High concentrations of nitric oxide in the distal esophagus have been observed in patients with GERD who have ingested nitrate.22
Table 44-1 Proposed Physiologic Abnormalities Contributing to Gastroesophageal Reflux Disease in Patients with Barrett’s Esophagus*
| ABNORMALITY | POTENTIAL CONSEQUENCES |
|---|---|
| Extreme LES hypotension | Gastroesophageal reflux |
| Ineffective esophageal motility | Defective clearance of refluxed material |
| Gastric acid hypersecretion | Reflux of highly acidic gastric juice |
| Duodenogastric reflux | Esophageal injury caused by reflux of bile acids and pancreatic enzymes |
| Decreased salivary secretion of epidermal growth factor | Delayed healing of reflux-damaged esophageal mucosa |
| Decreased esophageal pain sensitivity | Loss of sensation to refluxed caustic material and resulting failure to initiate therapy |
LES, lower esophageal sphincter.
* See Chapter 43 for detailed discussion of these abnormalities.
The progenitor cells that give rise to Barrett’s metaplasia are not known. The prevailing hypothesis is that metaplasia results when GERD damages the esophageal squamous epithelium, thereby exposing multipotential stem cells in the basal layers to gastric juice, which stimulates their abnormal differentiation into columnar cells. Two other candidates for Barrett’s progenitor cells include stem cells in the ducts of the esophageal submucosal glands and circulating bone marrow stem cells.23 Genes that appear to play a key role in the squamous-to-columnar metaplasia of Barrett’s esophagus include certain Cdx genes, which are known to mediate the differentiation of intestinal epithelial cells, and the gene encoding bone morphogenetic protein (BMP)-4, which also is involved in columnar cell differentiation.24 Reflux esophagitis appears to up-regulate the expression of these genes by the squamous epithelium.
Barrett’s epithelial cells appear to be more capable of resisting reflux-induced esophageal injury than the native squamous epithelial cells. Unlike squamous cells, for example, Barrett’s cells secrete mucins and express the tight-junction protein claudin 18, features that render the epithelium more resistant to acid-peptic attack.25,26 Unfortunately, Barrett’s epithelium also is predisposed to neoplasia.
MOLECULAR BIOLOGY OF NEOPLASIA
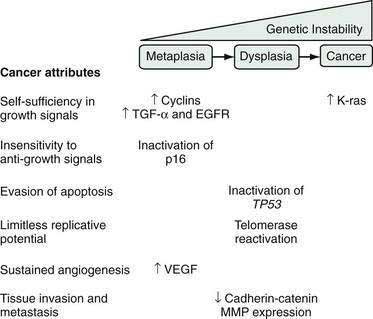
During carcinogenesis, Barrett’s epithelial cells accumulate a series of genetic and epigenetic alterations that endow the cells with the physiological attributes of malignancy (see Chapter 3). Those include self-sufficiency in growth signals, insensitivity to anti-growth signals, evasion of apoptosis, limitless replicative potential, sustained angiogenesis, and the abilities to invade adjacent structures and to metastasize (Fig. 44-2).27 Numerous genetic alterations have been described during the neoplastic progression of Barrett’s esophagus. Although a single such alteration may have multiple disparate effects, conceptually it can be useful to classify the alteration according to the major physiologic cancer attributes that it endows (see Fig. 44-2).27 For example, the expression of oncogenes (e.g., cyclin D1, K-ras), growth factors (e.g., transforming growth factor-α [TGF-α]), and growth factor receptors (e.g., epidermal growth factor receptor [EGFR]) enable Barrett’s cells to acquire self-sufficiency in growth signals. Insensitivity to antigrowth signals occurs primarily through the inactivation of tumor suppressor genes (e.g., TP53 and p16). Inactivation of TP53 also enables cells to evade apoptosis. Reactivation of the enzyme telomerase, which enables the cells to replace telomeres needed for cell division, can endow the cells with limitless replicative potential.18 Neoplasms can increase their vascular supply by secreting angiogenic factors such as vascular endothelial growth factor (VEGF).18 Finally, for neoplastic cells to invade and metastasize, they must dissociate themselves from surrounding cells by disrupting cell adhesion proteins such as the cadherins and catenins, and by degrading the extracellular matrix through the secretion of enzymes such as matrix metalloproteases (MMPs).18
During carcinogenesis, Barrett’s epithelial cells display genetic instability manifested as gains or losses in segments of chromosomes, which alter the cells’ deoxyribonucleic acid (DNA) content. Aneuploidy is the condition in which there is abnormal cellular DNA content, and aneuploid cells are at increased risk for neoplastic progression.28 Aneuploidy can be detected by flow cytometry and by fluorescence in situ hybridization (FISH), and has been proposed as a biomarker for neoplastic progression in Barrett’s esophagus, as have a number of the genetic alterations discussed in the preceding paragraph.29 Although there have been some promising preliminary studies, molecular biomarkers are not yet ready for routine clinical use in patients with Barrett’s esophagus.
DYSPLASIA
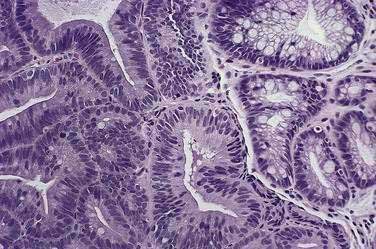
Before neoplastic Barrett’s cells become malignant, some of the same genetic alterations that endow the physiologic attributes of malignancy also cause morphologic changes in the tissue that the pathologist recognizes as dysplasia (Fig. 44-3). Dysplasia (also called intraepithelial neoplasia) can be viewed as the histologic expression of genetic alterations that favor unregulated cell growth.30 Dysplasia is recognized by cytologic and architectural abnormalities in esophageal biopsy specimens that include (1) nuclear changes such as enlargement, pleomorphism, hyperchromatism, stratification, and atypical mitoses; (2) loss of cytoplasmic maturation; and (3) crowding of tubules and villiform surfaces. Dysplasia is categorized as low-grade or high-grade depending on the degree of histologic abnormalities, with more pronounced abnormalities assumed to reflect more severe genetic damage and greater potential for carcinogenesis. Pathologists have difficulty distinguishing low-grade dysplasia in Barrett’s esophagus from reactive changes caused by reflux esophagitis, and inter-observer agreement for the diagnosis of low-grade dysplasia may be less than 50%. Interobserver agreement is better (approximately 85%) for high-grade dysplasia, but there is substantial disagreement among pathologists in distinguishing high-grade dysplasia from intramucosal carcinoma (see Chapter 46).
Dysplasia in Barrett’s esophagus often causes no endoscopically apparent abnormalities, and dysplasia can be patchy in its extent and severity. These factors contribute to the substantial problem of biopsy sampling error in identifying dysplasia. Although endoscopists traditionally have used a four-quadrant biopsy sampling system (essentially a random sampling technique) to find dysplasia in Barrett’s esophagus, this system can miss areas of dysplasia and even cancer. In series of patients who had esophagectomies because endoscopic examination with biopsies revealed high-grade dysplasia in Barrett’s esophagus, for example, a number of studies have found that invasive cancer is present in 30% to 40% of the resected esophagi.31 However, a critical review of those studies suggests that 13% is a more accurate estimate of the frequency of invasive cancer in this situation.32
Researchers have tried to develop endoscopic techniques for recognizing dysplasia and early cancer in Barrett’s esophagus including chromoendoscopy, autofluorescence endoscopy, magnification endoscopy, narrow band imaging, optical coherence tomography, Raman detection methods, and confocal laser endomicroscopy.33 There have been some promising preliminary results (see Chapter 46) but presently none of the techniques has provided sufficient clinical information to justify its routine application in clinical practice.
The overall incidence of cancer development in patients with Barrett’s esophagus is approximately 0.5% per year. A recent study suggests that patients who have non-neoplastic Barrett’s esophagus develop low-grade dysplasia at the rate of 4.3% per year, and high-grade dysplasia at the rate of 0.9% per year.34 Few meaningful data are available on the natural history of low-grade dysplasia in Barrett’s esophagus, but a recent study that included 156 patients with low-grade dysplasia found that they developed cancer at an incidence of 0.6% per year.35
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree