Because of the impaired inflammatory response caused by the immunosuppressive therapy, signs and symptoms of infection may be greatly attenuated. The consequences of this are several: a greater need for invasive biopsies, even in the face of unimpressive (although unexplained) lesions, and increased reliance on computed tomographic (CT) and/or magnetic resonance (MR) imaging in place of conventional radiography in patients with subtle signs and symptoms.
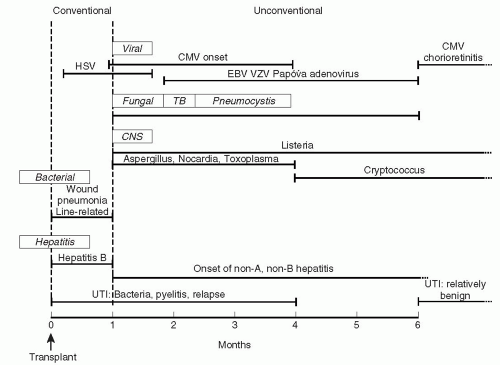
The potential effects of the impaired inflammatory response on the course of an infection are many (Fig. 26.1). When the clinical course is compared to that of a normal host, the transplant patient will usually have less pronounced symptoms for a sustained period, until there is a rapid deterioration which may be impossible to treat; the
microbial burden is significantly higher in the transplant patient, requiring more intensive and/or sustained courses of antibiotics, with attendant risks of antimicrobial resistance and toxicity; and, for those diseases like tuberculosis (TB) in which person-to-person transmission is important, the efficiency of spread is greatly increased.
The prognosis for a patient is significantly influenced by how early in the process diagnosis is made and therapy instituted. The tension between the need for early diagnosis and the attenuation of clinical clues caused by immunosuppression is the essence of transplant infectious disease.
The range of organisms capable of causing clinical disease in transplant recipients is quite large. They can be divided into three general categories: (a) true pathogens, (b) sometime pathogens, and (c) nonpathogens. True pathogens are the classic plagues of mankind (e.g., influenza, bubonic plague, typhoid); they cause disease by crossing fascial planes, invading normal tissue; and/or by producing toxins. Innate immunity is not able to control these infections, and survival is dependent on the development of specific immunity and/or the institution of effective antimicrobial therapy. Sometime pathogens are usually present on the mucocutaneous surfaces of the body (e.g., Staphylococcus aureus on the skin and Bacteroides fragilis and Escherichia coli on the gut mucosa). As long as these surfaces remain intact, the colonizing bacteria have little impact. Damage to the surfaces provides the means for tissue invasion, with these sometime pathogens, when delivered to the wrong site, being quite capable of causing significant disease. Nonpathogens of importance are typically commensal organisms in the environment (e.g., Aspergillus sp, Mucor sp, Rhizopus sp, Nocardia sp, etc.), unable to cause disease except in immunocompromised individuals. The term opportunistic infection is used to describe invasive infection caused by nonpathogens or life threatening infection caused by an organism that causes only trivial disease in the normal host.
Because the consequences of infection in these patients are potentially so great, the emphasis should be placed on prevention rather than treatment of established infection. Thus, there is great interest in the deployment of preemptive and prophylactic antimicrobial regimens (1).
exposures that the patient encounters; and the net state of immunosuppression.
TABLE 26.1. Environmental bacterial exposures | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
is a tenfold increase in the incidence of significant infection in those patients who are hypoalbuminemic (1,4,5).
TABLE 26.2. Factors contributing to the net state of immunosuppression | |||||||
|---|---|---|---|---|---|---|---|
|
Despite the importance of the issues related to donor and recipient related infection, it must be emphasized that approximately 95% of infections in the first month posttransplant are the same bacterial infections of the wound, lungs, urine, and bloodstream seen in nonimmunosuppressed patients undergoing comparable surgery. The chief determinant of the incidence of these infections is the skill with which the transplant operation is performed and the postoperative care accomplished. Antimicrobial prophylaxis, aimed at skin flora, for 1 to 3 days will improve things further, but nothing takes the place of technically impeccable management.
The first month posttransplant is characterized by two observations: the highest daily doses of immunosuppressive drugs, and a lack of opportunistic infection. Indeed, a single case of opportunistic infection during this one month “golden period” is prima facie evidence of a significant environmental exposure that must be identified and corrected. These observations also teach us that the prime determinant of the net state of immunosuppression is the sustained therapy (“the area under the curve”), not what was administered on a particular day.
Infection that was present in the recipient prior to transplant and that was not eradicated (a poor idea at best) prior to surgery and the initiation of immunosuppression—both of which will tend to amplify the extent and the impact of such infection. In the patient who has had multiple surgeries prior to the development of ESRD (typically, a child with significant congenital anatomic abnormalities), we have seen “cold abscesses” due to Staphylococcus aureus at a site of old wound infection reactivate posttransplant. More typically, the occurrence of aspiration pneumonia, peritonitis, and/or UTI merits attention prior to transplant. The biggest concern is bloodstream infection, as seeding of the vascular suture line can cause a mycotic aneurysm susceptible to catastrophic rupture.
Unrecognized bacterial infection in the donor can lead to devastating outcomes in the recipient, particularly if bloodstream infection is engendered. There are three potential sources of donor derived infection: the illness that led to organ donation could have been associated with bacteremic seeding; the organ could have been contaminated in the harvesting procedure; finally, most cadaveric donors have been subjected to critical care unit evaluation and treatment prior to organ donation, with all the attendant
risks from vascular access, urinary catheters, endotracheal tubes, etc. Thus, we have reported Pseudomonas sepsis of donor origin leading to vascular anastomotic infection and catastrophic rupture in both recipients of kidneys from a single donor (9). What was notable in this case was that the donor had no signs or symptoms of infection prior to organ harvest, although blood cultures from the donor were positive for the same organism at the time of organ donation. A report of methicillin-resistant Staphylococcus aureus infection in the recipients of the kidneys, liver, and cornea of a single donor highlights the issue of transmission of active infection from the donor to the recipients (10). Similarly, an outbreak of Pseudomonas aeruginosa infection in the recipients of a liver, kidney, kidney-pancreas, and lungs from a single donor has been reported, which resulted in infection and disruption of the vascular anastomosis (11). In this case, direct contamination of donor tissue (including harvested donor vessels) by Pseudomonas-laden sputum was probably the mechanism of infection.
The first consideration is the nature of the organism involved and the therapy that was prescribed up to this point. Some organisms with lesser virulence require a shorter duration and lower intensity of treatment prior to organ harvesting, provided there is evidence of clinical response (e.g., pneumococcal and/or meningococcal meningitis). In contrast, organisms that are virulent and tend to metastasize, particularly to endothelial surfaces, should be avoided. These include Staphylococcus aureus, Streptococcus pyogenes, Pseudomonas aeruginosa, and Salmonella sp.
Thus, organ transplantation may be considered if the infecting species is relatively bland (e.g., E. coli) or is rapidly cleared from the bloodstream with bactericidal antibiotics (penicillin-sensitive pneumococci and meningococci) for a minimum of 4 days, with rapid clearance of blood cultures following the initiation of therapy.
Organs from potential donors with bacteremia or invasive infection with the following organisms should not be used: Streptococcus pyogenes, vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus, Streptococcus milleri, Salmonella sp, Nocardia sp, or mycobacteria.
All patients receiving organs from a donor with recently treated bacteremia should receive bactericidal antibiotics directed against the donor’s isolate, and this should be continued for a minimum of 10 to 14 days posttransplant.
These recommendations should be regarded as tentative, and an international registry should be established in order to monitor the success and failure of this approach.
nephrectomy should be considered, especially if there is any concern for a vascular anastomotic leak, which could have fatal consequences (1).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree