Response criteria
Groups
Definition
Advantages
Disadvantages
References
Radiologic
RECIST
Complete response
Disappearance
– Objective, well-evaluated response measure in solid tumors
– Not well established in BRCP and LAPC
[12]
Partial response
30 % decrease
– Complete response rare in PDAC
Stable disease
Neither partial response nor progressive disease criteria met
– Bad correlation with surgical and pathologic endpoints
Progressive disease
20 % increase; no complete response, partial response, or stable disease documented before increased disease
NCCN resectability status
Resectable
Arterial:
– No arterial tumor contact
– Well defined, clinically relevant endpoint
– True radiologic downstaging rare
[9]
Venous:
– No tumor contact with the SMV or PV or ≤180° contact without vein irregularity
– Internationally accepted
– No correlation with resection rate and R0 status
Borderlinea
Arterial:
– Solid tumor contact with the CHA without extension to the celiac axis or hepatic artery bifurcation allowing for safe and complete resection and reconstruction
– Solid tumor contact with the SMA ≤180°
– Presence of variant arterial anatomy making resection possible
Venous:
– Solid tumor contact with the SMV or PV >180°, contact of ≤180° with contour irregularity of the vein or thrombosis of the vein but with suitable vessel proximal and distal the site allowing for safe and complete resection and vein reconstruction
– Solid tumor contact with IVC
Unresectablea
Arterial:
– Solid tumor contact with SMA >180°
– Solid tumor contact with CA >180°
– Solid tumor contact with first jejunal SMA branch
Venous:
– Unreconstructable SMV/PV due to tumor involvement or occlusion
Cancer stage
cT0
Primary tumor cannot be assessed
– Good correlation with pathologic T-staging possible
– True radiologic downstaging rare
[49]
cT1
Tumor limited to the pancreas, 2 cm or less in greatest diameter
– Limited data
cT2
Tumor limited to the pancreas, more than 2 cm in greatest diameter
cT3
Tumor extends beyond the pancreas but without involvement of the celiac axis or the superior mesenteric artery
cT4
Tumor involves the celiac axis or the superior mesenteric artery
Pattern of recurrence
Local recurrence
–
– Evaluation of local/distant efficacy of preoperative treatment
– Necessitating standardized radiologic follow-up
–
Peritoneal metastases
–
Liver metastases
–
Other distant metastases
–
Surgical
Resection rate
Number of resected patients/all patients
– Important of surgical success
Biased endpoint as definitions of resectable/unresectable frequently not described
–
Number of resected patients/explored patients
– Important prognostic endpoint, as resected patients have better survival than unresected
Indications for surgery/resection frequently unclear
– Does not correlate with radiologic response
Exploration rate
Number of explored patients/all patients
– indirect parameter of treatment toxicity
same as above
Pathologic
Margin status
R0
Microscopic tumor-free margin
– Most frequently described pathologic parameter
– Standardized pathologic handling necessary
R1
Microscopic residual tumor
– Prognostic relevance in PDAC (without pretreatment) established
– Different definitions of R0 between classifications
R2
Macroscopic residual tumor
– Description of tumor clearance at all margins (in mm) necessary
– No correlation with radiologic response
Complete pathologic response
pCR
No residual tumor left after neoadjuvant treatment
– Frequently described
– Rare event in PDAC
–
– Not useful as single response parameter
Evans score
Grade I
Little (<10 %) or no tumor cell destruction
– Standardized measure of tumor response
– Infrequent use
[60]
Grade IIa
Destruction of 10–50 % of tumor cells
Grade IIb
Destruction of 51–90 % of tumor cells
Grade III
Few (<10 %) viable-appearing tumor cells
Grade IV
No viable tumor cells
CAP Score
Grade 0
No viable residual tumor (=pathologic complete response, pCR)
– Semi-standardized measure of tumor response
– Infrequent use
[59]
Grade 1
Marked response (minimal residual cancer with single cells or small groups of cancer cells)
Grade 2
Moderate response (residual cancer outgrown by fibrosis)
Grade 3
Poor or no response (extensive residual cancer)
Biochemical
CA19-9
–
–
– High positive predictive value
– Low negative predictive value
– Not applicable in all PDAC patients
Survival
Overall survival
OS
In RCT time from randomization until death from any cause, measured in the intent-to-treat population
– Most objective endpoint
– Differences exist in the calculation of OS (initial diagnosis/beginning of preoperative treatment/from surgery)
– Unbiased
Disease-free survival
DFS
In RCT DFS is defined as the time from randomization until recurrence of tumor or death from any cause
– Surrogate for clinical benefit given the high morbidity of recurrence
– Less objective than OS
– Dependent on radiologic assessment
Treatment-related
Toxicity (CTCAE)
Grade 1
Mild; asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated
– Clinically important endpoint to assess toxicity of preoperative treatment
– Impractical for surgical adverse event assessment
[74]
Grade 2
Moderate; minimal, local or noninvasive intervention indicated; limiting age-appropriate instrumental activities of daily living
– Well defined for individual toxicities
Grade 3
Severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care activities of daily living
– Widely used
Grade 4
Life-threatening consequences; urgent intervention indicated
Grade 5
Death related to adverse event
Dindo-Clavien perioperative morbidity
Grade I
Any deviation from the normal postoperative course without the need for pharmacological treatment or surgical, endoscopic, and radiological interventions
– Widely used in surgery
– Time-consuming in surgeries with high morbidity
[79]
Grade II
Requiring pharmacological treatment with drugs other than such allowed for grade I complications. Blood transfusions and total parenteral nutrition are also included
– Objective well-defined endpoints
Grade III
Requiring surgical, endoscopic, or radiological intervention
Grade IIIa
Intervention not under general anesthesia
Grade IIIb
Intervention under general anesthesia
Grade IV
Life-threatening complication (including CNS complications) requiring IC/ICU management
Grade IVa
Single organ dysfunction (including dialysis)
Grade IVb
Multiorgan dysfunction
Grade V
Death of a patient
Radiologic Endpoints
While standardized definitions of BRPC and LAPC were lacking, in recent years the CT-based anatomic classification of BRPC developed at the M.D. Anderson Cancer Center has gained wide acceptance in the USA as it was adopted by the American Hepato-Pancreato-Biliary Association (AHPBA), the Society of Surgical Oncology (SSO), the Society for Surgery of the Alimentary Tract (SSAT), and the National Comprehensive Cancer Network (NCCN) since 2013 [9] (see section “Radiologic Endpoints”). This definition has recently been adopted by the International Study Group of Pancreatic Surgery (ISGPS) [10]. Prerequisite for this anatomic classification is a multi-detector, thin (submillimeter) CT scan with CT angiography using a pancreatic protocol, with images obtained in the portal venous, arterial, and pancreatic phase of contrast enhancement [9].
Given the CT-based primary staging of BRPC and LAPC, radiologic response evaluation following neoadjuvant treatment seems consequential. Although interobserver variability seems low in CT-based staging of pancreatic cancer [11], current data on this outcome metric is limited.
RECIST
Although Response Evaluation Criteria in Solid Tumors (RECIST) criteria [12] have been well established in other solid tumors, as well as, in metastatic PDAC, its usefulness in response assessment in BRPC/LAPC seems questionable (Table 9.1). In a meta-analysis of 111 trials including more 4394 patient undergoing neoadjuvant treatment for PDAC, only 6 studies explicitly used RECIST criteria for response evaluation and less than 40 % of trials clearly stated their criteria to assess tumor response [13]. Similarly, in trials specifically evaluating neoadjuvant therapy in BRPC, RECIST criteria are used in less than 50 % of cases [14]. In the above-mentioned meta-analysis, the CR rate of all trials (i.e., including those with unclear definitions) was just 3.8 % (95 %CI: 3–4.9 %) and the PR rate 29 % (95 %CI: 26–34 %). Katz et al. could show that in 122 well-characterized BRPC patients at a single-institution, complete remission (CR) following neoadjuvant therapy as defined by RECIST did not occur at all, and that partial response (PR) was rare, occurring in only 15 patients (12 %) [15]. Similar results have been reported by other groups [16, 17].
Resectability Status
Some studies have used the NCCN definitions of resectable, BRPC, LAPC, or metastatic disease to describe response to preoperative therapy. Similar to RECIST data, true radiologic downstaging seems to be a rare event and occurred in just 1 % of the aforementioned study by Katz et al. [15]. In contrast, disease progression and upstaging occurs in roughly 20 % of patients independent of the primary tumor classification [13, 15, 16]. Preliminary data indicate that tumor downstaging might be increased using novel regimes, including FOLFIRINOX and nab-Paclitaxel, which have shown high response rates in the metastatic setting [18–20]. However, radiologic imaging still does not correlate with surgical resection or pathologic response parameters [20] (Fig. 9.1).
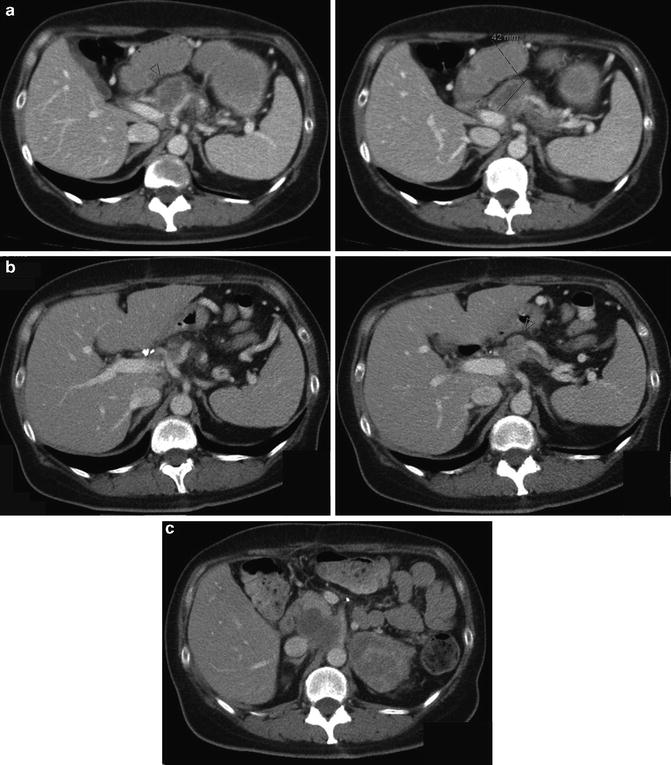

Fig. 9.1
A 53-year-old women with locally advanced adenocarcinoma of the pancreatic body. (a) Radiologic staging at primary diagnosis. The tumor was deemed unresectable following laparotomy and exploration due to infiltration of the celiac axis. (b) Restaging after 6 cycles of FOLFIRINOX. Radiologic response with tumor regression, but persistent signs of celiac axis infiltration. The patient underwent successful re-exploration and tumor resection with extended distal pancreatectomy, splenectomy, and partial resection of the common hepatic artery with end-to-end anastomosis of the common and proper hepatic arteries. Pathologic analysis revealed a margin-free tumor specimen with minimal clearance of <0.1 cm at the ventral resection margin: UICC-classification (7th edition, 2010): ypT3, ypN0 (0/29), L0, V0, Pn1, G3-4, R0, CRM+ (cranio-dorsal, <0.1 cm ventral), response grade 1 according to Evans. (c) Local recurrence 6 months after resection
Cancer Stage
Other unbiased measures of radiologic response evaluation like radiographic tumor stage following treatment (T-stage) (Table 9.1) or primary tumor diameter before and after preoperative therapy have rarely been reported [14]. Similar to the results of RECIST criteria, the objective response rates for these outcome metrics have been low in BRPC/LAPC [13, 16].
Positron Emission Tomography Imaging
Very limited data exists on positron emission tomography (PET) imaging for response assessment and it has not been tested in large prospective trials so far [21].
Pattern of Recurrence
As one of the main arguments in favor of preoperative therapy is the “sterilization” of the tumor bead and occult micrometastases the pattern of recurrence might be used as an important response criterion, but has as yet not been widely used [22]. The ability of PDAC to spread along nerve routes and ganglia causing high rates of local recurrence [23] might be reduced using preoperative regimes. Therefore, tight and standardized radiologic follow-up is recommended in clinical trials of preoperative therapies (Fig. 9.1).
Importantly, all radiologic response measures do not adequately predict surgical exploration and more important resection rates (see below) [13, 16, 24, 25] (Fig. 9.1). While diagnostic sensitivity for arterial involvement as one of the main features of LAPC has been described as high as 97 %, sensitivity has been poor with rates between 67 and 91 % [26–29]. The reason for the inadequate radiological staging of neoadjuvant-treated PDAC seems to be the extensive peritumoral desmoplastic and inflammatory reaction elicited by pancreatic tumor growth and the surrounding stroma [30]. Consequently, microscopic complete pathologic resections (R0) are more frequent than would have been expected by imaging and intraoperative findings (see below).
Until results from ongoing trials with high methodological quality, clear BRPC and LAPC definitions, and sound response classifications based on objective criteria are available [31], the clinical relevance of radiologic response evaluation is limited. Based on current data, radiologic imaging cannot be used to predict successful (R0) surgical resection and its clinical use is therefore limited [15, 17].
Surgical Endpoints
Resection Rate
Resection rates have widely been reported in studies investigating preoperative treatment of BRPC and LACP. In a recent meta-analysis, resection rate was 50.7 % (95 %CI: 44.0–57.4 %) in all patients (i.e., resectable and unresectable at primary diagnoses) undergoing neoadjuvant therapy. As expected resection rates were higher in patients deemed initially resectable (73.6 %; 95 %CI: 65.9–80.6 %) than in patients deemed unresectable at primary diagnosis (33.2 %; 95 %CI: 25.8–41.1 %). Similar rates have been reported in other meta-analysis focusing on BRPC patients [16], radiotherapy [32, 33], phase II trials [34], or specific chemotherapy regimes [35].
These results are frequently interpreted as arguments in favor of neoadjuvant therapy, but have to be interpreted with caution. Surgical resection rate is a highly biased endpoint for preoperative response assessment as: (a) most studies lack clear definitions of resectable, unresectable, BRCP, and LAPC, i.e., inclusion criteria are unclear; (b) the current absence of adequate radiologic staging tools to differentiate between neoplastic and non-neoplastic desmoplasia leads to non-validated treatment decisions; (c) clear indications for surgical exploration and resection are lacking. Therefore, the question of whether these results truly reflect downstaging due to neoadjuvant treatment or whether similar resection rates could have been achieved with direct surgery remains unclear. Results from a multicenter database study indicate that for BRPC with venous involvement only, neoadjuvant therapy does not increase resection rate [36] and is consequently discouraged in some guidelines [10]. Furthermore, as pointed out above, resection rates are generally higher than would have been expected from radiological response assessment. Hence, indication for surgical exploration should be handled liberally until better and more reliable imaging criteria are available.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







