TIPS: transjugular intrahepatic portosystemic shunt; NO: nitric oxide.
Therefore, on the basis of available evidence, it can be concluded that in patients with mild sodium retention, restriction of dietary sodium is probably not necessary; the hypothetical benefit of low salt diet in the achievement of a negative sodium balance is overridden by the marked natriuretic effect of diuretics. In contrast, in patients with marked sodium retention, who usually have a less intense natriuretic response to diuretics compared with patients with moderate sodium retention, dietary sodium restriction (80 mmol of sodium per day) may facilitate the elimination of ascites and delay the reaccumulation of fluid. A more severe restriction of sodium is not recommended because it is poorly accepted by patients and may impair their nutritional status. C5
Therapeutic paracentesis
Therapeutic paracentesis has progressively replaced diuretics as the treatment of choice in the management of patients with cirrhosis and large volume ascites [14, 15]. This change in treatment strategy is based on the results of several randomized controlled trials comparing paracentesis (either removal of all ascitic fluid in a single tap or repeated taps of 4–6 1iters/day) associated with plasma volume expansion versus diuretics [16–20]. Ald Because paracentesis does not affect renal sodium retention, patients should be given diuretics after paracentesis to avoid reaccumulation of fluid [21]. Ald
Two aspects concerning the use of therapeutic paracentesis in patients with cirrhosis and ascites deserve to be specifically discussed: (1) the population of patients with cirrhosis in whom therapeutic paracentesis should be used; and (2) the use of plasma expanders to prevent disturbances in circulatory function after paracentesis. While most physicians consider that therapeutic paracentesis is the treatment of choice for all patients with large volume ascites [14, 15], others believe that therapeutic paracentesis should be used only in those patients who show a poor or no response to diuretics [22]. Results of randomized trials indicate that therapeutic paracentesis is faster and in several trials was associated with lower incidence of adverse effects compared with diuretics (see Table 38.1) [16–20]. Ald Moreover, therapeutic paracentesis may have a better cost-effectiveness profile compared with diuretic treatment that can result in prolonged hospitalization. Therefore, on the basis of the available evidence, it seems clear that the use of therapeutic paracentesis should not be restricted to patients failing to respond to diuretics but should be considered the treatment of choice for all patients with large volume ascites (see Box 38.1).
The removal of large volumes of ascitic fluid is associated with circulatory dysfunction characterized by a reduction of effective blood volume [23–29]. Several lines of evidence indicate that this circulatory dysfunction and/or the mechanisms activated to maintain circulatory homeos-tasis have detrimental effects in cirrhotic patients. First, circulatory dysfunction is associated with rapid reaccumulation of ascites [29]. Second, approximately 20% of these patients develop hepatorenal syndrome and/or water retention leading to dilutional hyponatremia [23]. Third, portal pressure increases in patients developing circulatory dysfunction after paracentesis, probably owing to an increased intrahepatic resistance due to the action of vasoconstrictor systems on the hepatic vascular bed [27]. Finally, the development of circulatory dysfunction is associated with shortened survival [29].
Table 38.1 Adverse effects in randomized trials comparing the efficacy and safety of diuretics versus therapeutic paracentesis and plasma volume expansion in patients with cirrhosis and large volume ascitesa.
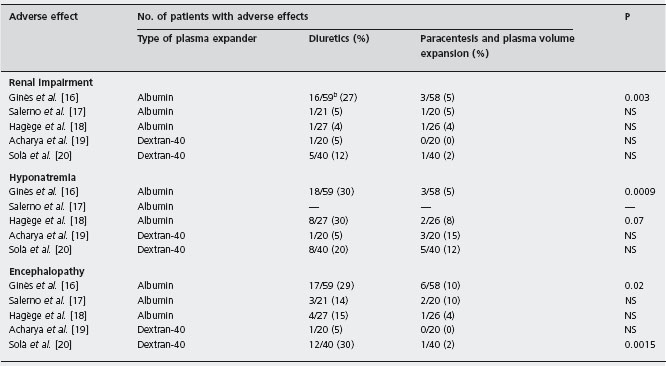
aDifferences in the rate of adverse effects among the studies may be due, at least in part, to differences in the populations of patients included.
bFigures represent the number of patients developing the adverse effects compared with the total number of patients in each treatment group.
BOX 38.1 Recommendations for the management of patients with cirrhosis and large volume ascites [1]
(1) Total paracentesis plus intravenous albumina (8g/l of ascites removed). Patients can be treated as outpatients. Hospitalization is recommended for patients with associated complications (i.e. encephalopathy, bacterial infection, gastrointestinal bleeding).
(2) After removal of ascitic fluid, start with moderate sodium restriction (80mmol/day) and diuretics, either aldosterone antagonists alone (i.e. spironolactone 50–400 mg/day) or in combination with loop diuretics (i.e. furosemide 20–100 mg/day). If patients were on diuretics before the development of large volume ascites, check compliance with sodium diet and diuretic therapy. Compliant patients should be given doses of diuretics higher than those given before paracentesis in order to prevent the recurrence of ascites. Non-compliant patients should be instructed to comply with therapy.
(3) Consider liver transplantation.
aAlthough a survival benefit of albumin over other plasma expanders has not been demonstrated, albumin is more effective than other plasma expanders in the prevention of paracentesis-induced circulatory dysfunction when more than 5l of ascitic fluid are removed.
The most studied effective method to prevent circulatory dysfunction is the administration of plasma expanders. A randomized trial has shown that albumin is more effective than other plasma expanders (dextran-70, polygeline) for the prevention of circulatory dysfunction as estimated by changes in plasma renin activity, probably owing to its persisting longer in the intravascular compartment [29]. Alc When less than five liters of ascites are removed, dextran-70, polygeline or saline show efficacy similar to that of albumin. However, albumin is more effective than these other plasma expanders when more than five liters of ascites are removed [29]. Alc Despite this greater efficacy, randomized trials have not shown differences in survival of patients treated with albumin compared with those treated with other plasma expanders [29–33]. Larger trials would be required to demonstrate a benefit of albumin on survival as well as on renal function. Table 38.2 shows the incidence of adverse effects observed in randomized trials comparing therapeutic paracentesis without plasma volume expansion or with three different plasma expanders in patients with cirrhosis and large ascites.
In summary, conclusive results from a randomized trial with adequate power to demonstrate a benefit of albumin administration after therapeutic paracentesis on mortality are not available. However, the currently available data indicate that circulatory dysfunction after removal of large amounts of ascitic fluid is potentially harmful to patients with cirrhosis. Albumin appears to be the plasma expander of choice when more than five liters of ascites are removed.
Table 38.2 Adverse effects reported in randomized trials assessing the efficacy and safety of therapeutic paracentesis without plasma volume expansion or with different plasma volume expanders in patients with cirrhosis and large ascites.
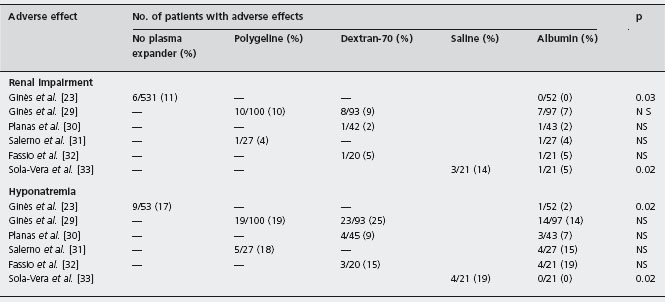
Figures represent the number of patients developing the adverse effect compared with the total number of patients in each treatment group.
Diuretics
Diuretics eliminate the excess extracellular fluid presenting as ascites and edema by increasing renal sodium excretion, thus achieving a negative sodium balance. The diuretics most frequently used in patients with cirrhosis and ascites are aldosterone antagonists, mainly spironolactone and potassium canrenoate, drugs that selectively antagonize the sodium-retaining effects of aldosterone in the renal collecting tubules, and loop diuretics, especially furosem-ide, that inhibit the Na+-K+-2Cl– co-transporter in the loop of Henle [34].
Despite the use of diuretics in clinical practice for more than 30 years, few randomized trials have been reported comparing the efficacy of different diuretic agents in the treatment of ascites [34–36]. In patients without renal failure, the aldosterone antagonist spironolactone at a dose of 150mg/day (increased to 300mg/day if there was no response) was shown in one randomized trial to be more effective than the loop diuretic furosemide at a dose of 80mg/day (increased to 160mg/day if there was no response) [35]. A1d This increased efficacy of aldosterone antagonists has also been suggested in several studies [13, 37–40]. Based on these findings, aldosterone antagonists are considered the diuretics of choice in the management of cirrhotic ascites.
In clinical practice, aldosterone antagonists are frequently given in combination with loop diuretics. Theoretical advantages of this combination include greater natriuretic potency, earlier onset of diuresis, and reduced tendency to induce hyperkalemia. Two different regimens of diuretic administration have been proposed. In the first, the dose of aldosterone antagonists is increased progressively (usually up to 400mg/day of spironolactone) and loop diuretics (furosemide up to 160mg/day) are added only if no response is achieved with the highest dose of spironolactone. In the second, the two drugs are given in combination from the start of therapy. Both regimens are similar with respect to efficacy and incidence of complications. The only difference is that when the combination of spironolactone and furosemide is used from the beginning of therapy there is a more frequent need to reduce the dose of the drugs in responsive patients compared with the other stepwise regimen [41]. Ald Diuretic therapy is effective in the elimination of ascites in 80–90% of all patients, a percentage that may increase to 95% when only patients without renal failure are considered [13,16–020, 35–40]. Ald B4 The remaining patients either do not respond to diuretic therapy or develop diuretic-induced adverse effects that prevent the use of high doses of these drugs. This condition is known as refractory ascites [42]. These adverse effects include hepatic encephalopathy, hyponatremia, renal impairment, potassium disturbances, gynecomastia and muscle cramps [34,40–43]. The incidence of renal and electrolyte disorders and encephalopathy vary depending on the population of patients studied, and is higher in patients with marked sodium retention and renal failure (who require higher doses of diuretics) and lower in patients with moderate sodium retention and without renal failure. Although some of these complications may be unrelated to diuretic therapy and due to the existence of advanced liver disease [44], there is no doubt that diuretics are a major cause of these complications because their frequency is markedly lower if ascites is removed by therapeutic para-centesis (see Table 38.1). Spironolactone-induced gynecomastia is common and may be important enough to lead to the discontinuation of the drug in some patients. An alternative treatment for these patients is amiloride, although its potency is much lower than that of spironol-actone [36]. Ald Eplerenone, a new aldosterone antagonist has fewer endocrine adverse effects compared with spironolactone and could be a good alternative to spironol-actone in patients with spironolactone-induced gynecomastia [45]. However, its effectiveness in patients with cirrhosis and ascites has not been assessed. Finally, muscle cramps of variable intensity, sometimes severe, may also occur as an adverse effect of diuretics. Effective therapies for muscle cramps include quinidine (300 mg/day) [46], or albumin (25g/week) [43]. Zinc sulfate (440mg/day) also appeared effective in an uncontrolled study including a small number of patients [47]. B4
Because therapeutic paracentesis has replaced diuretics as the treatment of choice for hospitalized cirrhotic patients with large volume ascites in most centers, at present the main indications for use of diuretics in cirrhosis are as follows [15]:
- Treatment of patients with mild or moderate ascites or those with large volume ascites in whom paracentesis is not effective because of compartmentalization of ascitic fluid due to peritoneal adhesions.
- Treatment of patients with edema without ascites.
- Prevention of recurrence of ascites after therapeutic paracentesis.
Peritoneovenous shunt
A peritoneovenous shunt is a device designed to transfer ascitic fluid from the abdominal cavity to the systemic circulation via an abdominal tube and a thoracic tube ending in the superior vena cava connected through a one-way valve. This device was used extensively in the 1970s and 1980s for the treatment of refractory ascites in cirrhosis. Although the system was pathophysiologically sound, its use declined progressively during the 1990s due to a high incidence of severe adverse effects, a high rate of obstruction, lack of demonstration of a significant survival benefit, and development of new procedures, such as the transjugu-lar intrahepatic portosystemic shunt (TIPS) [48–54]. For all these reasons, this procedure is rarely used nowadays.
Transjugular intrahepatic portosystemic shunt
TIPS was introduced in clinical practice in the 1990s for the management of refractory variceal bleeding, with the objective of creating a portosystemic shunt, without the need of surgery. The procedure consists of the placement of an intrahepatic stent between one hepatic vein and the portal vein using a transjugular approach [55]. It soon became evident that in patients with variceal bleeding and ascites treated with TIPS there was an increased natriuretic effect of diuretics, leading to the reduction or elimination of ascites in most patients. These beneficial effects of TIPS on ascites are similar to those reported in earlier studies in patients treated with surgical portosystemic shunts, especially side-to-side portacaval shunts.
A large number of uncontrolled studies have shown that TIPS is effective in preventing recurrence of ascites in patients with refractory ascites. This effect is due to reduction in the activity of sodium-retaining mechanisms and amelioration of renal function, which lead to an improvement of the renal response to diuretics [56–61]. B4 The main disadvantages of TIPS include shunt stenosis or obstruction (up to 75% of patients develop stenosis within 6–12 months, leading to reaccumulation of ascites in most cases), but this has been reduced with covered stents. In addition, there is a high rate of encephalopathy due to the shunting of blood from the splanchnic to the systemic circulation [62, 63]. Other adverse effects include impairment in liver function, which is usually transient, hemolytic anemia and heart failure [55,64]. Because of its efficacy and the paucity of good alternative therapies (except for that of repeated large-volume paracentesis with concomitant administration of intravenous albumin), TIPS became a widely used treatment for patients with refractory ascites during the 1990s despite the initial lack of randomized controlled trials comparing it with medical therapy.
Five randomized trials comparing uncovered TIPS versus repeated large volume paracentesis with concomitant intravenous albumin in patients with cirrhosis and refractory ascites have been published [65–69]. Alc The trials demonstrate that TIPS controls ascites effectively and is associated with a lower rate of ascites recurrence. The main results of these trials are summarized in Table 38.3. Although there are some discrepancies between the results of the trials these studies demonstrate the following:
- TIPS is clearly more effective than large volume paracentesis in the prevention of recurrence of ascites [65–69]. However, normalization of renal sodium homeostasis is not completely achieved and most patients treated with TIPS still require sodium restriction and diuretics during follow-up [67–68].
- TIPS reduces the risk of developing hepatorenal syn drome type 1 [67].
- TIPS is associated with an increased risk of severe hepatic encephalopathy and does not reduce significantly the risk of other complications of cirrhosis, such as gastrointestinal bleeding or spontaneous bacterial peritonitis [67,68].
- There is a high rate of TIPS stenosis or obstruction that requires frequent intervention to maintain shunt patency [65–69].
- Hepatic encephalopathy after TIPS occurs in approxi mately 30–50% of patients [65–69].
- Despite better control of ascites and a reduction in the number of hospitalizations for ascites, TIPS does not appear to improve the quality of life compared with repeated large volume paracentesis with concomitant intravenous albumin [67,68].
- The cost of TIPS is higher than that of conventional therapy with repeated large volume paracentesis and concomitant intravenous albumin [67].
- TIPS does not improve either overall or transplant- free survival compared with therapy with repeated large volume paracentesis with intravenous albumin [67, 68, 70].
Table 38.3 Complications and survival in randomized trials comparing transjugular intrahepatic portosystemic shunt (TIPS) and large volume paracentesis in patients with cirrhosis and refractory ascites.
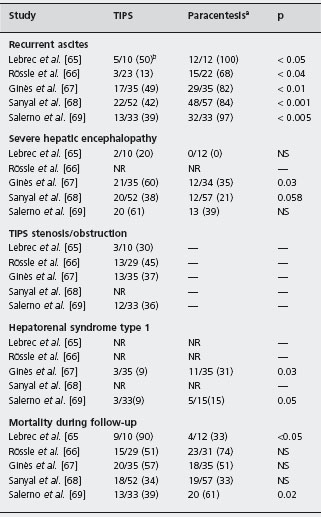
aIn all studies, except that of Rössle et al. [66] IV albumin (6–8 g/l ascites removed) was given routinely to all patients treated with paracentesis.
b Patients/total number of patients included.
NR: not reported; IV: intravenous.
A high quality meta-analyses of these randomized controlled studies concluded that TIPS is better at controlling ascites but does not improve survival compared with paracentesis [70]. A1c However, another recent meta-analysis that reviewed individual patient data of three trials indicates that TIPS significantly improves transplant-free survival of cirrhotic patients with refractory ascites [66, 68, 69, 71]. A1c Although meta-analysis using individual data seems a more reliable tool compared with standard meta-analysis, it cannot overcome deficiencies of individual studies, such as inclusion of patients who did not have refractory ascites in two of the studies [66,69], use of large-volume paracentesis without albumin in one study [66], and use of TIPS as rescue therapy in a large proportion of patients treated with large-volume paracentesis in another study [69]. Until more information becomes available, the higher frequency of hepatic encephalopathy as well as the higher cost of TIPS compared with large-volume paracentesis plus albumin, in the context of a lack of survival benefit, leaves TIPS as a second-line therapy in the management of refractory ascites. The first line of treatment for refractory ascites is repeated large-volume paracentesis associated with albumin [14,15]. TIPS placement may be an option for patients with preserved liver function (bilirubin < 3mg/dl, serum sodium level > 130mEq/l, Child-Pugh score < 12, model for end-stage liver disease (MELD) score < 18), aged < 70, without hepatic encephalopathy, central hepato-cellular carcinoma, or cardiopulmonary disease.
The recommendations for the treatment of refractory ascites based on these conclusions are summarized in Box 38.2.
Liver transplantation
Liver transplantation has become a frequent intervention for patients with advanced cirrhosis. Although randomized trials comparing liver transplantation with conventional medical therapy in patients with ascites are not available for obvious reasons, the 80% five-year probability of survival obtained in adult cirrhotic patients treated with liver transplantation in most centers is markedly greater than the expected 20% in non-transplanted patients with cirrhosis and ascites [72]. B2
BOX 38.2 Recommendations for the management of patients with cirrhosis and refractory ascites
(1) Total paracentesis plus intravenous albumin (8g/l of ascites removed). Repeat paracentesis during follow-up whenever needed. Patients can be treated as outpatients. Consider liver transplantation.
(2) Patients should be on sodium restriction (80mmol/day) and maximum tolerated closes of diuretics (up to spironolactone 400mg/day and furosemide 160 mg/ day). Check urine sodium under diuretic therapy. If urine sodium is greater than 30mmol/day, diuretic therapy may be maintained because it may help to delay the recurrence of ascites. If urine sodium is lower than 30mmol/day or diuretic treatment induces complications, diuretics should be withdrawn.
(3) Consider the use, of transjugular intrahepatic portosystemic shunt (TIPS) in patients with preserved liver function and with low acceptance of repeated total paracentesis or in those in whom paracentesis is not effective because of the presence of peritoneal adhesions.
Earlier recommendations suggested that ascites per se was not an indication for liver transplantation, and patients had to be considered for transplantation only when ascites was refractory to diuretic therapy or was associated with severe complications, such as spontaneous bacterial peritonitis or hepatorenal syndrome. However, with these guidelines a large proportion of these patients die while registered on the transplantation waiting list. This is because of the short survival associated with these conditions. The median survival time is less than one year for patients with refractory ascites and those recovering from spontaneous bacterial peritonitis, and is even shorter in patients with hepatorenal syndrome, particularly in those with the progressive form of this syndrome – type 1 – who have a median survival time of less than one month [50, 51, 73].
With the growing understanding of the natural history of ascites in cirrhosis, it is now known that a number of factors predictive of survival can be used to identify candidates for liver transplantation [74]. The most useful predictive factors are related to abnormalities in renal function and systemic hemodynamics and include: • An impaired ability to excrete a water load (urine volume < 8 ml/min after a water load of 5% dextrose 20 ml/ kg intravenous (IV)).
- Spontaneous dilutional hyponatremia (serum sodium < 130mmol/l).
- Arterial hypotension (mean arterial pressure < 80 mmHg in the absence of diuretic therapy).
- Reduced glomerular filtration rate (even moderate reductions, as indicated by serum creatinine levels between 1.2 (106μmol/l and 1.5mg/dl (133μmol/l) in the absence of diuretic therapy).
- Marked sodium retention (urine sodium < 10mmol/day under a moderate sodium-restricted diet and in the absence of diuretic therapy).
Interestingly, in patients with ascites these parameters are better than liver function tests as predictors of prognosis [74]. Therefore, patients with one or more of these predictive factors have a poor survival expectancy and should be referred to transplant centers for evaluation. B4
The MELD score (Model for End-stage Liver Disease score, which includes serum bilirubin, international normalized ratio (INR) and serum creatinine) may be suitable for the evaluation of prognosis of patients with cirrhosis and ascites, as it includes a variable that estimates the degree of impairment of renal function [75]. In addition, many patients with advanced cirrhosis and refractory ascites may have low serum sodium (levels less than 130mEql/l), which is an independent poor prognostic factor for outcome and survival. In fact both the MELD score and the serum sodium concentration are independent predictors of mortality. Thus, a new score incorporating MELD and serum sodium (MELD-NA) has been proposed, since serum sodium improves the prognostic accuracy of the MELD score in the prediction of survival in these patients [76, 77]. Nevertheless, the inclusion of serum sodium in a new score for survival prediction in cirrhosis has some limitations given that serum sodium is a very labile parameter. In addition, this new score needs to be validated in prospective studies.
Hepatorenal syndrome
Hepatorenal syndrome is at the most severe end of the clinical spectrum of abnormalities of renal function in patients with cirrhosis and ascites [3, 6, 42, 78]. It may occur in two different clinical patterns [42, 78]. Type 1 hepatorenal syndrome is characterized by rapid and progressive impairment of renal function as defined by a 100% increase of the initial serum creatinine to a level greater than 2.5mg/dl (221μmol/l) or a 50% reduction of the initial 24-hour creatinine clearance to a level lower than 20ml/min in less than two weeks; in some patients, this type of hepatorenal syndrome develops spontaneously without any identifiable precipitating factor, while in others it occurs in close chronological relationship with some complicating event, particularly in relation to bacterial infections such as spontaneous bacterial peritonitis [79].
BOX 38.3 Diagnostic criteria of hepatorenal syndrome
(1) Cirrhosis with ascites.
(2) Serum creatinine > 1.5mg/dl (133μmol/l).
(3) No improvement of serum creatinine (decrease to a level lower than 1.5mg/dl–133μmol/l– after at least two days off diuretics and volume expansion with albumin (1 g/kg body weight up to a maximum of 100 g/day).
(4) Absence of shock.
(5) No current or recent treatment with nephrotoxic drugs.
(6) Absence of signs of parenchymal renal disease, as suggested by proteinuria (>500mg/day) or hematuria (<50 red blood cells per high power field), and/or abnormal renal ultrasound.
Adapted from Salerno et al. Gut 2007; 56: 1310–1318.
Type 2 hepatorenal syndrome is characterized by a less severe and non-progressive reduction of glomerular filtration rate (at least in the short term); the main clinical consequence of this type of hepatorenal syndrome is refractory ascites.
Because of the lack of specific diagnostic tests, the diagnosis of hepatorenal syndrome is currently made according to several criteria, as proposed by the International Ascites Club, which are based on demonstration of a marked reduction in glomerular filtration rate (serum creatinine > 1.5mg/dl in the absence of diuretic therapy) and the exclusion of other causes of renal failure that may occur in patients with cirrhosis (see Box 38.3) [42, 78].
For many years, no effective therapy existed for patients with hepatorenal syndrome, except for liver transplantation. Recently, several effective, new interventions have been introduced.
Vasoconstrictors
A number of observational studies published in the 1990s showed that the administration of vasoconstrictor drugs to patients with cirrhosis and hepatorenal syndrome causes a marked improvement of renal function in a large proportion of patients [80–92]. B4, C5 The rationale for the use of vasoconstrictors in patients with hepatorenal syndrome is to improve effective arterial blood volume by causing a vasoconstriction of the extremely dilated splanchnic vascular bed. The improvement in the arterial circulatory function leads to a suppression in the activity of vasoconstrictor systems and a subsequent increase in renal perfusion and glomerular filtration rate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







