Once the AV access has been in use, the most important factors that limit its survival are stenosis, thrombosis, and infection. In general, complications occur more commonly in grafts than in AV fistulas.
I.
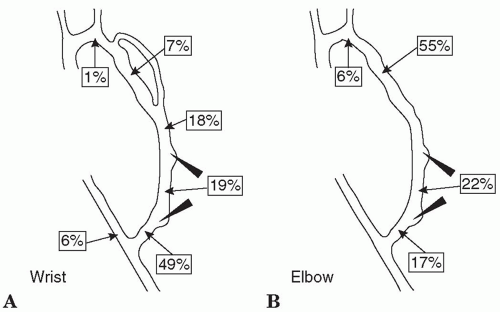
STENOSIS. Vascular access stenosis is a harbinger of thrombosis, reduces access blood flow, and can lead to underdialysis. The most common cause of stenosis in AV grafts is neointimal hyperplasia, which usually occurs at or just distal to the graft-vein anastomosis. In AV fistulas, the location and cause of stenosis is more varied, with the juxta-anastomotic region being a frequent site. Common sites of stenosis in AV fistulas and grafts are shown in
Figures 8.1 and
8.2. Because access patency is much worse after thrombectomy than after elective angioplasty, current KDOQI guidelines recommend prospective monitoring and surveillance of AV fistulas and grafts for hemodynamically significant stenosis. Not all guidelines recommend routine monitoring, however, and there is controversy regarding the overall clinical benefit of maintaining an access surveillance program (
Kumbar, 2012;
Paulson, 2012). Randomized controlled trials have not consistently shown that surveillance improves outcomes in grafts; in fistulas, surveillance has been shown to reduce the rate of thrombosis, but may not prolong overall fistula life.
There are several strategies to detect stenosis prior to definitive visualization of the access tract by Doppler ultrasound and, in the case of central vein stenosis, by venography. These early detection strategies depend on indirectly observing access pressure, flow, or recirculation during dialysis. The optimum early detection strategy differs somewhat for fistulas versus grafts, and for forearm versus upper arm locations. The basic principles are these: (a) Recirculation of dialyzed blood across the access device immediately back through the dialysis circuit does not appear until access flow decreases to a level near to or less than flow in the extracorporeal circuit. Thus, barring inadvertent needle reversal or improper needle placement, access recirculation will not be present until access flow falls to the range of 350-500 mL/min. At this range of flow, AV grafts are already at high risk for thrombosis,
so if true recirculation is detected in an AV graft, it is an urgent indication to image the graft and correct the stenosis. On the other hand, in AV fistulas, continued patency is likely even when recirculation is present (flow in the 350-500-mL/min range). The benefits of screening AV fistulas for access recirculation are relatively small in terms of preventing thrombosis, but screening for recirculation is useful to prevent underdialysis. Access stenosis that occurs between the usual sites of needle insertion will not cause recirculation, but may markedly reduce access flow to thrombosis-prone levels. Stenosis in this location should be suspected when access flows are measured to be below the blood pump flow rate, but recirculation is not detectable. (b) Both grafts and fistulas commonly develop inflow stenosis, so strategies that detect inflow stenosis will be useful for both types of AV access. (c) Outflow stenosis occurs much more frequently in grafts than in forearm fistulas where the degree of neointimal hyperplasia is less and where accessory outflow veins often compensate for obstruction of a principal outflow channel. However, in upper arm fistulas, outflow stenosis is not uncommon. Hence, strategies that detect outflow stenosis will be more useful in monitoring function of AV grafts and of upper arm fistulas.
A.
Physical examination of an AV access was discussed in some detail in
Chapter 6.
Table 8.1 shows the changes in physical findings with some common access complications. Physical examination can be quite useful in detecting isolated inflow or outflow access stenoses, but is less effective in detecting combined inflow and outflow lesions. The accuracy of physical examination is substantially higher if the persons doing the examination have received special training (
Coentrão, 2012). The ESRD Network of Texas has sponsored some training documents and examples, which are available on the Web (
Beathard, 2012).
B. Access surveillance using information obtained routinely during every dialysis session. Many dialysis machines have the option of measuring in vivo ionic dialysance. In all dialysis machines, the outflow venous pressure is monitored. Trending the results of these measurements over time can help detect access stenosis.
1.
Trending ionic dialysance. The ionic dialysance measured via conductivity includes any access recirculation component if present; as the degree of access recirculation increases, the in vivo ionic dialysance will decrease, assuming that other features of the dialysis prescription (dialyzer
K0A, blood and dialysate flow rates, heparinization) are kept constant. Dialysis machines that measure ionic dialysance typically integrate the clearances measured during each treatment (
K) to calculate a treatment
Kt value (clearance × time) for that session. In one case series of six patients with AV fistulas, a sustained fall in
Kt of 20% was associated with access recirculation (
Fontseré, 2011). Another approach is to follow the ratio of ionic dialysance to blood flow. In one report, a ratio of ≤0.5 had a high sensitivity and specificity for access recirculation (
Mohan, 2010).
2.
Trending venous outflow pressures. Venous pressures are measured continuously during routine hemodialysis. Venous pressures are a function of needle size, hematocrit (by its effect on blood viscosity), and blood flow rate. All other things being equal, a progressive rise in venous pressure over time (weeks to months) is often due to access outflow stenosis (
Zasuwa, 2010). Some large dialysis organization data systems are able to track such pressures and trend them over time, and one company in the United States (Vasc-Alert, Lafayette, IN) sells software that allows easy access to trended pressure data. One can also trend prepump arterial pressure, which will increase (in a negative direction) with worsening access inflow stenosis.
The sensitivity of pressure measurements during dialysis to detect access stenosis can be increased by focusing on measurements taken at the beginning of dialysis with the blood flow rate set at a low value (200-225 mL/min), because at high blood flow rates, much of the resistance to flow is from the needle and not the vascular access. A baseline pressure value should be established when the access is first used. The threshold pressure that triggers further evaluation depends on the size of the needle, blood viscosity, and other factors; for 15G needles, a starting venous pressure threshold to use might be >115-120 mm Hg; for 16G needles, the threshold might be >150 mm Hg. Such threshold pressures must be exceeded on three or more treatments in succession to be significant.
C. Periodic measurements of access blood flow rate. To what extent a low access flow rate reflects stenosis and an increased risk of thrombosis depends on the type of access. Flow through a forearm AV fistula commonly averages 500-800 mL/min, and in grafts, flow is somewhat higher, about 1,000 mL/min. Flow in upper arm fistulas or grafts may be considerably higher. AV fistulas may maintain patency at flows as low as 200 mL/min, whereas AV grafts begin to clot at access flows between 600 and 800 mL/min— flows that often provide adequate dialysis but offer few clinical premonitory signs that the access is at risk for thrombosis. The current KDOQI (2006) recommendations are to have the patient referred for access visualization if access flow is <600 mL/min or if the access flow is <1,000 mL/min and has decreased by >25% over the preceding 4 months. While regular surveillance of vascular access for stenosis has been shown to decrease thrombosis rates when compared with historical controls, recent prospective studies have not shown conclusively that detection of stenosis and correction with angioplasty improves graft survival.
1.
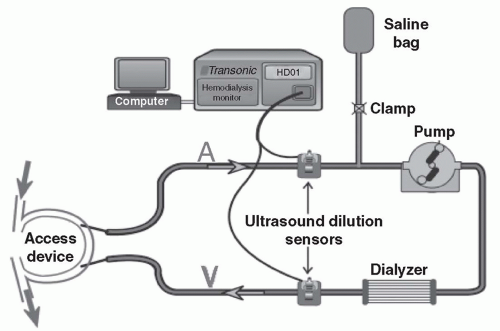
Direct measurement of access flow by saline dilution. This method for measuring access blood flow during hemodialysis treatments was pioneered by
Krivitski (1995). The required equipment is made by Transonic Systems, Inc. (Ithaca, NY) and consists of a control box, two matched flow/dilution sensors, a laptop computer, a data analysis software package, and a rolling stand that can be easily moved between patients (
Fig 8.3). The
setup shown in
Fig. 8.3 is for measurement of access recirculation; hence the needles are not reversed. To measure access blood flow, one must deliberately cause access recirculation in the extracorporeal blood circuit by reversing the arterial and venous lines so that the dialyzer is fed from the downstream access needle (
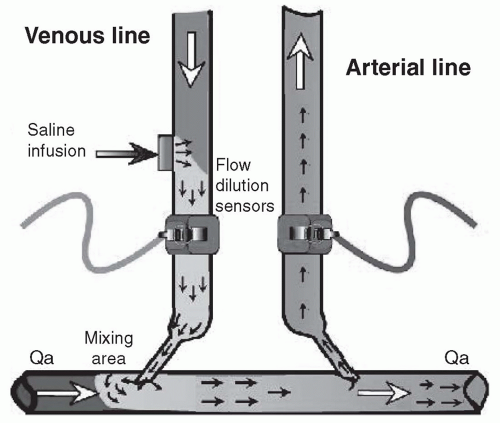
Fig 8.4). The degree of recirculation in such a system will depend on the ratio of the access blood flow rate to the rate of blood flow through the dialyzer. If the percentage recirculation and blood flow rate through the dialyzer are known, the access blood flow rate can then be calculated.
To measure the amount (percentage) of recirculation under needle-reversed conditions, a bolus of saline is injected into the blood leaving the dialyzer (
Fig. 8.4). The amount of dilution in the outlet bloodline is measured by a downstream ultrasound sensor. The speed of sound through blood depends on the concentration of proteins in the plasma; accordingly, the dilution effect of the saline bolus in the outlet bloodline can be quantified using this first sensor. Some of this saline-diluted blood will then traverse the vascular access segment between the two needles and reappear at the dialyzer inlet. The proportion of the saline-diluted blood that reappears at the dialyzer inlet depends on the ratio of access blood flow to the blood flow through the dialyzer. A second ultrasound sensor on the bloodline leading to the dialyzer is then used to detect the proportion of saline-diluted blood that reappears at the dialyzer inlet (
Fig. 8.4). In practice, an additional measurement of recirculation is made with the blood lines not reversed, as the presence of any recirculation under nonreversed conditions will affect the calculations.