I.
BLOOD CLOTTING IN THE EXTRACORPOREAL CIRCUIT. The patient’s blood is exposed to intravenous cannulas, tubing, drip chambers, headers, potting compound, and dialysis membranes during the dialysis procedure. These surfaces exhibit a variable degree of thrombogenicity and may initiate clotting of blood, especially in conjunction with exposure of blood to air in drip chambers. The resulting thrombus formation may be significant enough to cause occlusion and malfunction of the extracorporeal circuit. Clot formation in the extracorporeal circuit begins with activation of leukocytes and platelets, leading to surface blebbing and shedding of surface membrane lipid-rich microparticles, which initiate thrombin generation, activation of coagulation cascades, further thrombin formation and fibrin deposition. Factors favoring clotting are listed in
Table 14.1.
A. Assessing coagulation during dialysis
1.
Visual inspection. Signs of extracorporeal circuit clotting are listed in
Table 14.2. Visualization of the circuit can be best accomplished by rinsing the system with saline solution while temporarily occluding the blood inlet.
2.
Extracorporeal circuit pressures. Arterial and venous pressure readings may change as a result of clotting in the extracorporeal circuit, depending on the location of thrombus formation. An advantage of using blood lines with a postpump arterial pressure monitor is that the difference between the postpump and venous pressure readings can serve as an indicator of the location of the clotting. An increased
pressure difference is seen when the clotting is confined to the dialyzer itself (increased postpump pressure, decreased venous pressure). If the clotting is occurring in or distal to the venous blood chamber, then the postpump and venous pressure readings are increased in tandem. If the clotting is extensive, then the rise in pressure readings will be precipitous. A clotted or malpositioned venous needle also results in increased pressure readings.
3. Dialyzer appearance after dialysis. The presence of a few clotted fibers is not unusual, and the headers often collect small blood clots or whitish deposits (especially in patients with hyperlipidemia). More significant dialyzer clotting should be recorded by the dialysis staff to serve as a clinical parameter for adjustment of anticoagulant dosing. It is useful to classify the amount of clotting on the basis of the visually estimated percentage of clotted fibers in order to standardize documentation (e.g., <10% of fibers clotted, grade 1; <50% clotted, grade 2; >50% clotted, grade 3).
4. Measurement of residual dialyzer volume. In units practicing dialyzer reuse, automated or manual methods are used to determine the clotting-associated fiber loss during each
treatment. This is done by comparing the predialysis and postdialysis fiber bundle volumes. Dialyzers suitable for reuse characteristically have <1% fiber loss over each of the first 5-10 reuses.
II.
USE OF ANTICOAGULANTS DURING DIALYSIS. When no anticoagulant is used, dialyzer clotting rate during a 3- to 4-hour dialysis session is substantial (5-10%), and when this occurs, it results in loss of the dialyzer and blood tubings, plus loss of approximately 100-180 mL of blood (the combined fill volume of the dialyzer and blood line in the extracorporeal circuit). This is an acceptable risk in many patients judged to be at moderate to high risk of anticoagulant-induced bleeding, because bleeding in such patients may often result in catastrophic consequences, and for such patients anticoagulation-free dialysis (described below) can be appropriately used. However, for the great majority of patients, who are judged not to be at a markedly increased bleeding risk, some form of anticoagulation must be employed. In programs
reusing dialyzers, proper levels of anticoagulation during dialysis are key to obtaining reasonable reuse numbers.
There is considerable variability among regions of the world, countries, and even dialysis units about what type of anticoagulation is used during intermittent hemodialysis. Despite a number of promising alternatives, heparin remains the most common anticoagulant used. In the United States, unfractionated heparin is mostly used, whereas in the European Union, low-molecular-weight heparin (LMWH) is the anticoagulant of choice recommended by the
European Best Practice Guidelines (2002). A small number of dialysis units anticoagulate the blood circuit using trisodium citrate, and in special circumstances, direct thrombin inhibitors such as argatroban, heparinoids (danaparoid, fondaparinux), prostanoids, and nafamostat maleate may be used as alternative anticoagulants.
III. MEASURING BLOOD CLOTTING DURING DIALYSIS. While it is important to understand the principles of how clotting tests can be used to monitor heparin therapy, in the United States, because of economic constraints, the relatively low risk of bleeding complications from use of heparin during dialysis, and regulatory issues (the requirement for local laboratory certification), heparin therapy is ordinarily prescribed empirically, without monitoring of coagulation. In patients who are at an elevated risk of bleeding, the need to monitor anticoagulation is often circumvented by using heparin-free dialysis.
When clotting studies are done, blood for clotting studies should be drawn from the arterial blood line, proximal to any heparin infusion site, to reflect the clotting status of the patient rather than that of the extracorporeal circuit. It is very difficult to obtain baseline clotting studies from a venous catheter that has been locked with heparin, because of problems of residual heparin in the catheter, and this step is rarely attempted (
Hemmelder, 2003).
A. Clotting tests used to monitor heparin therapy
1.
Activated partial thromboplastin time (APTT). This is for unfractionated heparin monitoring only. This is the most commonly used test in a hospital setting. APTT results vary with individual assays, so many centers report a ratio compared to control (APPTr). Heparin resistance states can be falsely suggested owing to elevated levels of factor VIII. Baseline levels may be prolonged because of lupus anticoagulant (
Olson, 1998).
2. Whole-blood partial thromboplastin time (WBPTT). This is similar to above, but is a bedside test. The WBPTT test accelerates the clotting process by addition of 0.2 mL of actin FS reagent (Thrombofax) to 0.4 mL of blood. The mixture is set in a heating block at 37°C for 30 seconds and then tilted every 5 seconds until a clot forms. The prolongation of the WBPTT is linearly related to the blood heparin concentration (in the range applicable to dialysis). It should not be used to monitor LMWH therapy.
3. Activated clotting time (ACT). The ACT test is similar to the WBPTT test but uses siliceous earth to accelerate the clotting process. ACT is less reproducible than WBPTT, especially at low blood heparin levels. Devices that automatically tilt the tube and detect clot formation facilitate standardization and reproducibility of both WBPTT and ACT. It is for unfractionated heparin monitoring only.
4. Lee-White clotting time (LWCT). The Lee-White test is performed by adding 0.4 mL of blood to a glass tube and inverting the tube every 30 seconds until the blood clots. Usually, the blood is kept at room temperature. Disadvantages of the LWCT test include the long period of time required before clotting occurs, extensive use of technician time required, and the relatively poor standardization and reproducibility of the test. LWCT is the least desirable method of monitoring clotting during hemodialysis. This test is now rarely used.
5. Activated Factor Xa. Factor Xa can be measured by chromogenic or functional clotting assays. Laboratory assays of anti-Xa activity differ, as some contain exogenous purified antithrombin (AT), and anti-Xa activity measured using these assays may not necessarily correlate with the biological effect (Greeves, 2002). Although unfractionated heparin can be monitored by Xa activity, this is typically reserved for LMWHs and heparinoids, typically aiming for a peak anti-Xa activity of 0.4-0.6 IU/mL, and <0.2 IU/mL at the end or shortly after completion of dialysis.
6.
Factor Xa-activated ACT. This test has been proposed as a more sensitive test for monitoring anticoagulation during use of LMWH (
Frank, 2004), but is not widely used in clinical practice.
IV. ANTICOAGULATION TECHNIQUES
A. Unfractionated heparin
1. Mechanisms of action. Heparin changes the conformation of AT, leading to rapid inactivation of coagulation factors, in particular, factor IIa. Unfortunately, heparin does stimulate platelet aggregation and activation, but these undesirable effects are counterbalanced by interference with binding and activation of coagulation factors at the platelet membrane. Undesired side effects of heparin include pruritus, allergy including anaphylactoid reactions, alopecia, osteoporosis, hyperlipidemia, thrombocytopenia, and excessive bleeding.
2.
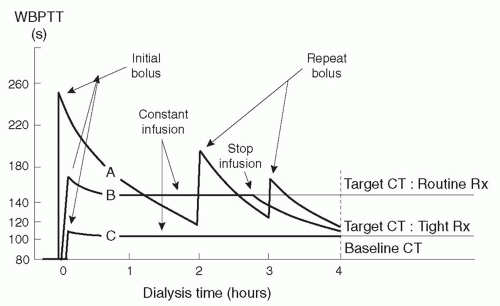
Target clotting times. Heparin can usually be given liberally during dialysis without fear of precipitating a bleeding episode in patients who do not exhibit an abnormal bleeding risk. The effect of two routine heparin regimens on clotting time is shown in
Figure 14.1. The goal is to maintain WBPTT or ACT at the baseline value plus 80% during most of the dialysis session (
Table 14.3). However, at the end of the
session, the clotting time should be shorter (baseline plus 40% for WBPTT or ACT) to minimize the risk of bleeding from the access site after withdrawal of the access needles.
The target clotting times using the Lee-White test are also listed in
Table 14.3. With LWCT, in contrast to WBPTT or ACT, the target clotting times during dialysis are considerably greater than baseline plus 80%, and the target LWCT values at the end of the session are greater than baseline plus 40%.
3. Routine heparin prescriptions. There are two basic techniques of administering routine heparin. In one method, a heparin bolus is followed by a constant heparin infusion. In the second, a heparin bolus is followed by repeated bolus doses as necessary. For the purpose of discussion, we present a typical prescription in each category.
Rx: Routine heparin, constant-infusion method
Administer the initial bolus dose (e.g., 2,000 units). The initial heparin dose is best administered to the patient via the venous access tubing and flushed in with saline (rather than being infused into the arterial blood line). Introducing heparin into the arterial blood line means that incoming nonheparinized blood will need to be pumped through the dialyzer until the loading dose has had time to pass through the extracorporeal circuit to anticoagulate blood in the body. Wait 3-5 minutes to allow heparin dispersion before initiating dialysis.
Start heparin infusion into the arterial blood line (e.g., at a rate of 1,200 units per hour).
Rx: Routine heparin, single-dose-only or repeated-bolus method
Administer the initial bolus dose (e.g., 4,000 units).
Then give an additional 1,000- to 2,000-unit bolus dose if necessary.
The prescriptions used in the United States, however, vary quite widely. Those centers that reuse dialyzers tend to use more heparin in order to maximize reuse number. Some centers give only a single initial dose (e.g., 2,000 units) of heparin, with no subsequent infusion or boluses. Some centers give a fairly large (75-100 units/kg) initial bolus dose followed by a 500- to 750-unit-per-hour infusion. At this point in time, there has been little research to convincingly demonstrate an optimal method of heparin dosing (
Brunet, 2008).
a.
Effect of body weight on the size of the heparin dose. Although in a population pharmacokinetic study the volume of distribution of heparin has been found to increase as body weight rises (
Smith, 1998), many dialysis centers do not regularly adjust heparin dosage in accordance with body weights ranging between 50 and 90 kg. Some centers do adjust both the loading and maintenance doses according to body weight.