Primary retroperitoneal
Secondary retroperitoneal
Kidneys
Head and body pancreas
Adrenal glands
Duodenum
Ureter
Ascending colon
Bladder
Descending colon
Aorta
Inferior caval vein
The anatomy of the retroperitoneum can be further divided by means of the renal fascia into the perirenal space (between anterior and posterior leaf of the renal fascia), which contains adrenal glands, kidneys, and renal vessels; the anterior renal space (between peritoneum and anterior leaf of the renal fascia) containing pancreas, colon, and duodenum; the posterior renal space (between posterior leaf of renal fascia and fascia transversalis) containing fat. The extraperitoneal paravesical pelvic space is beyond the scope of this chapter.
Imaging is important because the retroperitoneal space is difficult to access with bedside modalities such as auscultation, palpation, or percussion. Symptoms and signs may be obscure, delayed, nonspecific, or misleading. Moreover, the peritoneal cavity reacts more acutely and severely than the retroperitoneal tissues.
Imaging Techniques
Cross-sectional imaging techniques, such as ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI), dramatically improved the ability to evaluate the retroperitoneum. Selection of the optimal technique in each individual patient with a retroperitoneal mass is essential, and factors such as cost, radiation dose, and need for sedation should all be considered. Conventional radiography is of limited value, although it is the best technique to identify teeth in tumors with calcifications, which is virtually pathognomonic for mature teratomas. The initial modality to evaluate retroperitoneal masses is US because it is fast, inexpensive, has no radiation dose, and offers the possibility of fine-needle aspiration to confirm the infectious nature of a fluid collection or to perform histological biopsies in case of solid tumors. MRI and CT are not screening methods but are very useful in detailing osseous and soft tissue changes whenever conventional radiographs and US are not conclusive. CT provides good definition of possible bone destruction or sclerosis. The major drawback of CT is the radiation load. MRI is superior in defining soft tissue masses, bone marrow changes, cartilage destruction, and evaluating extension to the spinal canal (e.g., in neuroblastoma). The major drawback of MRI is the need for sedation in children ⩽6 years. Scintigraphy, with specific radiopharmacy, has additional value in determining the nature of certain tumors and evaluating metastases. Positron emission tomography (PET)-CT and total-body MRI are promising and are valuable in lymphoma evaluation.
Children versus Adults
Children are not small adults. There are significant anatomical, physiological, and psychological differences; and a large number of congenital and hereditary diseases may be added to the list of differences. Many of those differences have their adult radiological counterparts; therefore, it is not surprising that radiology in children is quite different from radiology in adults. This is true for the central nervous system (CNS), digestive tract, respiratory tract, urogenital tract, and retroperitoneal lesions, the latter being the topic of this chapter. Neonates are easy to handle, and all noninvasive imaging techniques can be used.
US is the initial modality in neonates for many clinical questions. The diagnostic value of US increases when the neonate is relaxed. Always use warm echogel, and in emergency cases, offering the infant a pacifier with syrup should do the trick.
Even MRI without anesthesia is feasible when performed immediately after feeding. Usually, newborn infants fall into a deep sleep after a meal, and this effect can be enhanced by some sleep and food deprivation prior to the meal. Intravenous access should be provided several hours prior to examination. However, this protocol fails after the age of 3–6 months. Older infants and preschool children usually need sedation for dedicated MRI examinations, although some clinical questions (control of hydrocephalus) can be answered with fast scanning techniques without the need for sedation.
CT techniques have been improved (dual-source CT), and scanning times <1 s are possible. Breathholding and motion lose their significance, and often radiation dose is much lower.
Once children go to school, their communication skills improve, and their voice should not be neglected by speaking only with the parents. However, do not even try to negotiate with a child (e.g., the amount of barium they have to drink): you will loose.
This chapter provides a brief overview of pediatric retroperitoneal pathologies in children.
Pancreas
Pancreatic masses are discussed separately in pp. 271–278 of the Kangaroo section of this syllabus.
Kidneys
Nephroblastomatosis
Nephroblastomatosis is defined as the presence of foci of persistent embryological metanephric blastema (nephrogenic rests), which have the potential (1–5%) to develop into nephroblastomas (Wilms’ tumor). Nephroblastomatosis presents as multinodular, peripheral, cortical lesions or as a subcapsular rind-like renal mass. On imaging, they are homogeneous and of low echogenicity on US, low density on CT, and low intensity on MRI. They are best appreciated on contrast-enhanced CT or T1-weighted MRI. Best diagnostic clue is its homogeneity.
Wilms’ Tumor
Wilms’ tumor (nephroblastoma) is the most common pediatric abdominal tumor, with a peak incidence at 3–4 years of age. It contains blastemal, stromal, and epithelial elements. Patients typically present with a painless unilateral abdominal mass. The tumors are often very large, and the first task of the radiologist is to determine the organ of origin (Fig. 1). Also, the renal vein and inferior caval vein must be evaluated because of the tendency of Wilms’ tumors to develop tumor extension into these veins and, rarely, into the ureter.
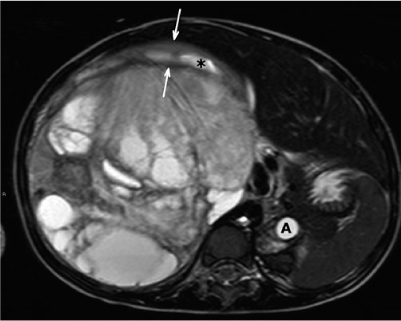

Fig. 1
Transverse T2-weighted magnetic resonance (MR) image. A 3-year-old girl with a right-sided palpable mass. Large Wilms’ tumor with cystic components causes displacement of the aorta (A). A small rim of stretched, normal parenchyma of the upper pole can be recognized (arrows). The asterisk marks a dilated calyx
Imaging shows a large, heterogeneous, nephrogenic tumor that may contain cysts, calcifications, and fatty elements. The collecting system is stretched along the tumor, and other retroperitoneal structures, such as the aorta and inferior caval vein, are displaced. There is no encasement and no elevation of the aorta (features of neuroblastoma). Also, retroperitoneal lymph nodes need to be examined for lymphogenic spread. Hematogenous metastasis has a predilection for the lungs. Staging of Wilms’ tumors is given in Table 2.
Table 2
Children’s Oncology Group (COG) staging of Wilms’ tumor (summarized)
Stage | Description |
|---|---|
I | Limited to kidney; completely resectable with intact renal capsule |
II | Infiltration beyond kidney; completely resectable |
III | Residual; confined to abdomen without hematogenous metastasis |
IV | Hematogenous spread |
V | Bilateral disease |
The contralateral kidney needs close inspection, because 4–13% of children have bilateral Wilms’ tumor, particularly in some syndromes that are known to be associated with the risk of developing Wilms’ tumor: Beckwith-Wiedemann syndrome and hemihypertrophy syndrome (both 5% risk), sporadic aniridia (30–40% risk), Drash syndrome, Perlman syndrome, Wilms’ tumor, aniridia, genitourinary anomalies, and retardation (WARG) syndrome, and Fanconi anemia. In these patients, screening is performed from 6 months until 7 years every 3 months with US.
Other nephrogenic tumors are less frequent, like the following.
Mesoblastic Nephroma
Mesoblastic nephroma commonly presents in the neonatal period (90% within first year of life) and is sometimes referred to as infantile Wilms’ tumor, although histology (spindle cells) does not resemble Wilms’ tumor. On imaging, it typically involves the renal sinus, is predominantly solid, and may contain cysts. These tumors seldom metastasize, and the prognosis is very good.
Multilocular Cystic Renal Tumor
Multilocular cystic renal tumor (multilocular cystic nephroma) is a predominantly cystic tumor. If the cyst wall contains blastemic elements, it is called a cystic partially differentiated nephroblastoma; if the cyst wall is well differentiated, it is called cystic nephroma. There are two age peaks: in children aged 3 months to 4 years (predominantly boys with cystic partially differentiated nephroblastoma), and in adults (predominantly women with cystic nephroma). The prognosis is good.
Rhabdoid Tumor
The rhabdoid tumor is considered a separate entity (it was formerly considered as a sarcomatous variant of Wilms’ tumor) occurring in a slightly younger age group (80% in children <2 years, 25% between 6 and 12 months). Imaging findings resemble Wilms’ tumor; however, it has other distinctive features. It has the worst prognosis of all childhood nephrogenic tumors and is often associated with primary or metastatic CNS lesions. Therefore, MRI of the brain is mandatory once histology is known.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








