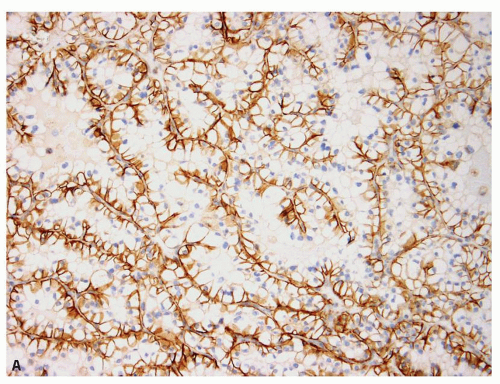
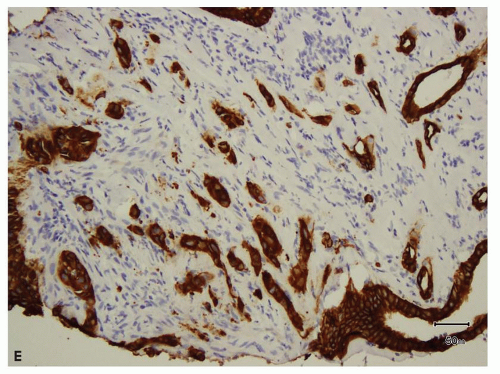
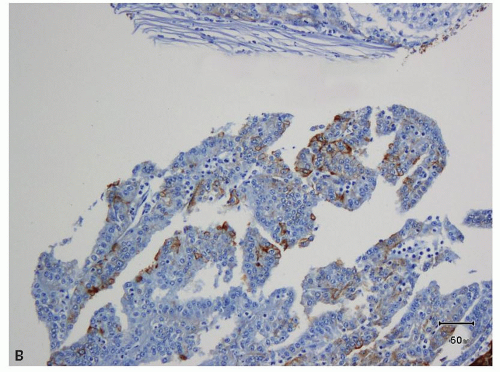
areas in most tumorous and nontumorous tissues (eFig. 13.2A,B), biopsies showing tumor necrosis merit interpretation with particular caution; perinecrotic areas almost always show strong membranous positivity for CA-IX (Fig. 13.4A,B). Thus, interpretation is quite problematic in biopsies with predominantly necrotic tumors in which the few viable areas often show diffuse membranous positivity. In such cases, the diagnosis of clear cell RCC cannot be rendered with certainty. Similarly, patchy staining in a biopsy— which may be a result of tumor ischemia—does not support the diagnosis of a clear cell RCC. Occasionally, CA-IX may show granular cytoplasmic staining in some tumors, mostly in those with eosinophilic cytoplasm (Fig. 13.5). Because CA-IX is a transmembrane molecule, cytoplasmic staining without membranous positivity is nonspecific and should not be regarded as a positive result. Among the renal cell tumors, CA-IX is also diffusely expressed in clear cell papillary RCC; however, the staining pattern is usually quite different (cup-shaped in clear cell papillary versus box-shaped in clear cell RCC) (13) (Fig. 13.6A,B, eFig. 13.3A,B). The other caveat is that CA-IX is often expressed in a diffuse, membranous pattern in mesotheliomas (10,11) and may also be expressed in urothelial carcinomas (14). Therefore, correlation with morphology, clinical history, and usage of other markers in the form of a panel is mandatory (Table 13.2). Most of the non-clear cell RCC that express CA-IX show positivity in a patchy manner and often will also show immunoreactivity for markers appropriate for that tumor.
TABLE 13.1 List of Antibodies Used in the Diagnosis of Renal Tumors | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 13.2 Most Useful, Limited Differentiating Immunohistochemical Panels for Tumors in the Kidneya | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
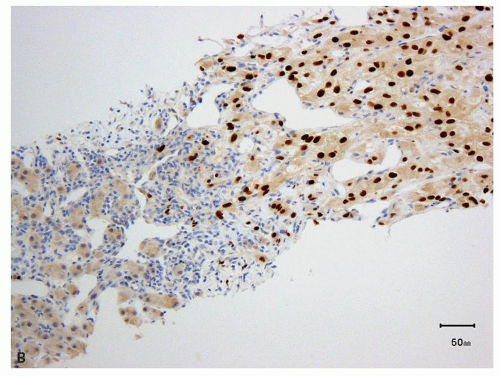
sites (Fig. 13.7A,B, eFigs. 13.4A,B) (15,16,17,18,19,20,21,22,23,24). Therefore, these are very useful markers for establishing renal cell origin in the proper clinical context. Overall, PAX8 appears to be a more sensitive marker than PAX2 (23), but in rare instances, the reverse may be true (22). Although of great utility for determining renal origin of a tumor, both the markers may also be expressed in the tumors of female genital tract (18,22), clear cell
adenocarcinoma of the bladder (25,26), peritoneal mesotheliomas (27), urothelial carcinomas—particularly of the upper tract (24), and thymic tumors (28). PAX8 is also uniformly positive in the tumors of thyroid. At the same time, staining for PAX8 and PAX2 may be absent in some tumors of renal cell origin, particularly high-grade tumors such as collecting duct carcinoma, sarcomatoid RCCs, and tumors with rhabdoid features;
chromophobe RCCs and renal oncocytomas may also show negative staining (22). Thus, positive staining in isolation cannot be regarded as definitive for renal cell origin, nor does the lack of staining exclude a renal origin. Proper interpretation requires analysis in association with other appropriate stains (Table 13.2).
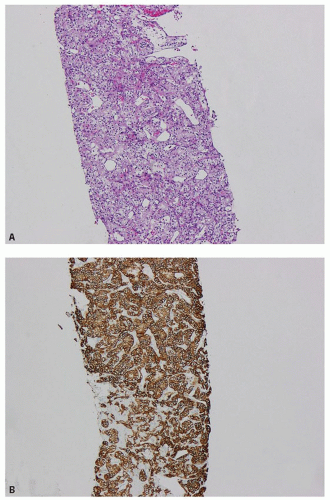
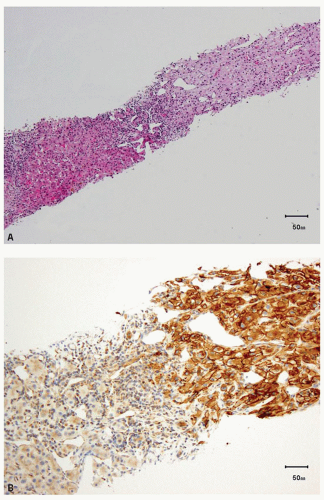 FIGURE 13.2 A: Clear cell RCC metastatic to liver (hematoxylin and eosin). B: CA-IX immunoreactivity in the metastatic tumor. |
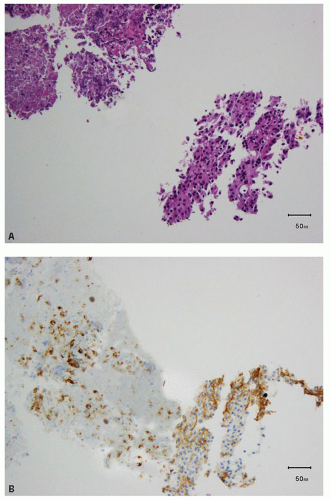
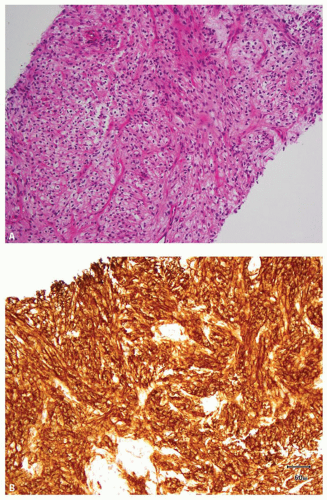 FIGURE 13.3 A: Spindle cell component of a sarcomatoid clear cell RCC. B: Strong, diffuse positivity for CA-IX in the sarcomatoid component of the tumor. |
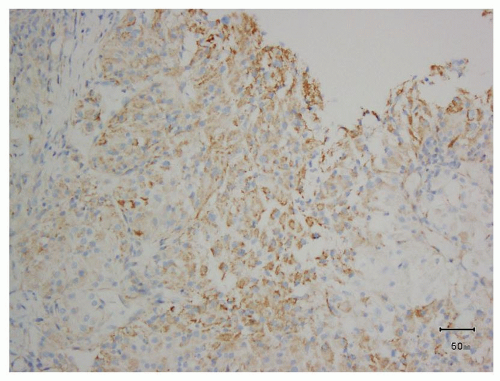 FIGURE 13.5 Granular cytoplasmic immunoreactivity without membrane accentuation should not be regarded as a positive result for CA-IX. |
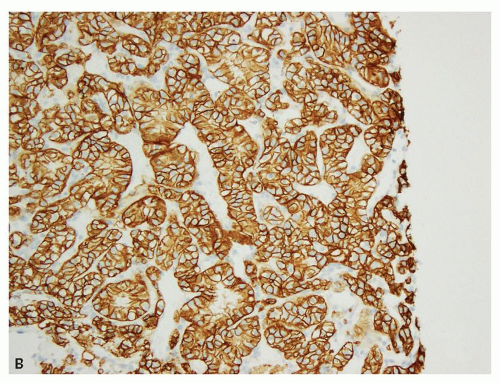
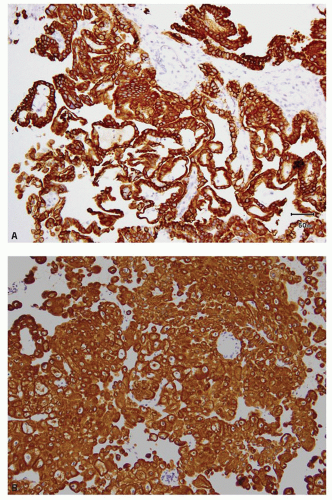
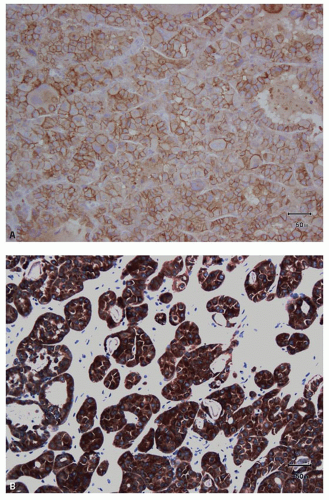
and leucocytes (29). It has been widely used as a marker for clear cell RCC in its differentiation from other renal tumors in both the primary and metastatic settings (30,31,32,33,34,35,36,37,38,39,40). In the appropriate clinicopathologic setting, diffuse membranous positivity is supportive of clear cell subtype among all the RCCs (Fig. 13.8A). However, diffuse cytoplasmic staining can be seen in a number of renal and nonrenal tumors (36) and is not helpful in this distinction. At the same time, membranous reactivity is also not specific for clear cell RCC and may be present in papillary RCC (Fig. 13.8B), colorectal, pancreatic, prostatic, and some vascular tumors (30,36,41). Both benign hepatic parenchyma and hepatocellular carcinoma show canalicular pattern of staining with CD10 (42). This may be mistaken for membranous staining, particularly in the limited material of needle core biopsies or cytologic preparations. Of import, clear cell papillary RCC is often negative for CD10, in spite of being positive for some other markers of clear cell RCC (13,43,44) (Tables 13.1 and 13.2). Thus in spite of its proven utility in clear cell RCC, given its nonspecificity, CD10 should not be used in isolation for the diagnosis of clear cell RCC and should only be used as a part of immunohistochemical panel (Table 13.2).
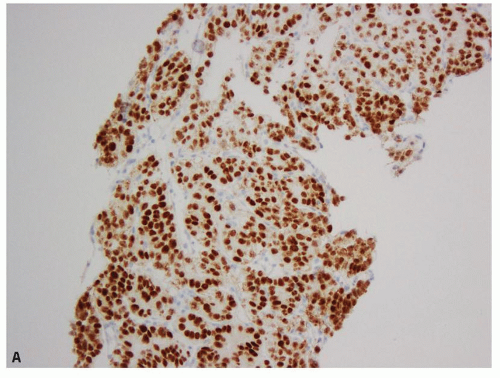 FIGURE 13.7 A: PAX8 showing diffuse nuclear positivity in a primary RCC. B: The immunoreactivity for PAX8 is retained at metastatic sites (clear cell RCC metastatic to the liver). |
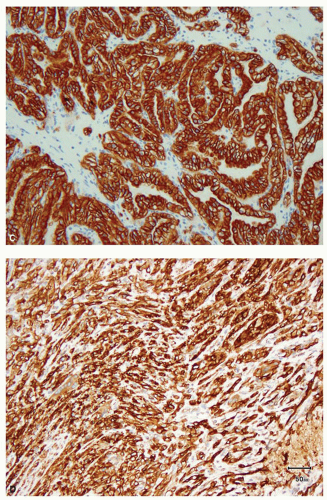
RCC (Fig. 13.9A), classical chromophobe RCC (Fig. 13.9B), clear cellpapillary RCC (Fig. 13.9C), mucinous tubular and spindle cell carcinoma (Fig. 13.9D), and urothelial carcinoma (6,13,42,44,45,46,47). However, papillary RCC type 2 and eosinophilic chromophobe show very variable results and may be negative or only focally positive for CK7 (48) (Fig. 13.10A,B).
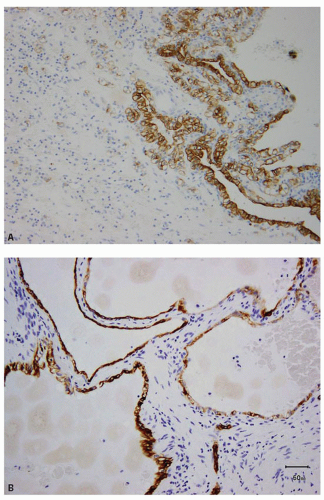
Clear cell RCC, with some rare exceptions, is usually completely negative for CK7, although cystic clear cell RCC often shows patchy positivity around the cysts. CK7 cannot be used in the distinction of clear cell papillary RCC from multilocular cystic RCC, as the latter also shows diffuse immunoreactivity for CK7 (Fig. 13.11A,B) (49). Renal oncocytoma is usually
negative or shows positivity in only rare dispersed cells. Metanephric adenoma is also usually negative for CK7 unlike type 1 papillary RCC, a close differential diagnostic consideration, particularly on limited biopsy material (50). In other renal tumors, results with CK7 are variable and inconsistent. Overall, CK7 remains a useful antibody in the differentiation of a number of renal tumors. However, one cannot completely depend on the results with this antibody alone in the distinction of renal oncocytoma from eosinophilic chromophobe RCC, or papillary type 2 RCC from other high-grade RCCs with prominent papillary architecture, particularly on biopsy specimens.
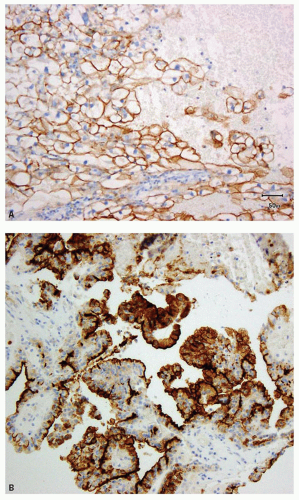 FIGURE 13.8 A: Diffuse membranous positivity for CD10 in a clear cell RCC. B: Papillary RCC showing the characteristic, predominantly luminal staining. |
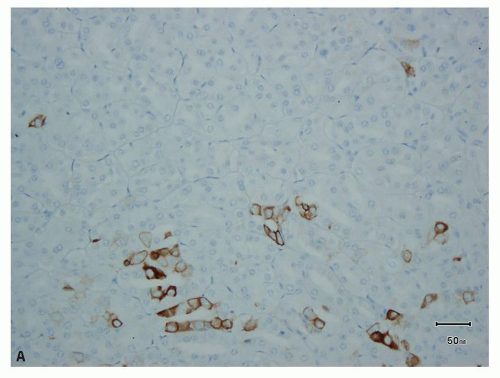 FIGURE 13.10 Only focal positivity for CK7 in (A) eosinophilic chromophobe and (B) type 2 papillary RCC. |
a number of nonrenal tumors, including hepatocellular carcinoma, lung adenocarcinoma, breast lobular carcinoma, ovarian clear cell carcinoma, testicular yolk sac tumor, and parathyroid carcinoma, among others, stain positive with the antibody (52). It is also expressed in greater than 40% of nonrenal tumors with papillary architecture (53). Given the availability of more sensitive and specific markers now, RCC Ma appears to have only limited utility in the diagnosis and differential diagnosis of renal tumors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree