Fig. 22.1
Photograph of a lesion in a patient who sought a second opinion day before starting chemoradiation therapy for an anal cancer. I did not feel the lesion was an anal canal lesion. It was a perianal lesion and it was locally excised. Gynecological oncologists would consider this lesion vulvar. The patient is disease-free 12 years later
Lack of Adoption
Finally, there has been a reluctance to learn HRA, a technique that allows for early detection and treatment of presumed premalignant lesions [6–8]. The premalignant lesions, HSIL, can be seen under direct visualization with either a colposcope or an operating microscope when the anal canal and distal rectal mucosa are treated with acetic acid. The technique is simple, can be done in the office or operating room, and uses equipment available in the operating room and in some offices. More importantly, this has been shown to decrease rates of progression to anal cancer in high-risk patients compared to historical controls of progression in average-risk patients and patients of undeclared risk [9–12]. And yet, the technique has not been widely adopted, with many citing a lack of evidence that HSIL progresses to cancer and concerns regarding the inability to accurately determine which patients need to be treated versus those that might otherwise be observed. Unfortunately for nonbelievers and more importantly HSIL patients, we now have data that untreated HSIL progresses to anal cancer [13].
The lack of adoption of HRA as a means for treating HSIL and preventing anal cancer raises the question as to why it hasn’t been more broadly accepted? During the time that HRA has been advocated but not adopted by most, laparoscopic colectomy was adopted as the standard of care, despite confounding variables that make it difficult to attribute the benefits claimed for laparoscopy to laparoscopy [14–17].
Concomitant with the widespread take off of minimally invasive colectomy, we witnessed the institution of care pathways that feed patients earlier, remove urinary catheters earlier, manage pain differently, and set expectations of an earlier discharge. This has led to shorter lengths of stay and earlier return to work in both open and laparoscopic procedures [14–18]. Currently, robotic surgery and single-incision surgery are being advocated and advanced similarly despite absence of trials proving benefit [19].
The argument that we shouldn’t treat anal HSIL because we don’t know which lesion will progress to anal cancer, or because it is a “field defect” that is therefore untreatable and the cancer will develop somewhere else, is inconsistent with the rest of much of our colorectal and medical practice patterns, in general. We treat esophageal dysplasia without knowing which region will progress to cancer. Similarly we treat cervical dysplasia, bile duct dysplasia, and colonic dysplasia with procedures that are high risk and highly morbid. These are all diseases with a “field defect” like that seen in anal dysplasia. In all of these diseases, we currently lack the knowledge that would allow us to predict who will progress and yet we treat and in many cases, aggressively. For some reason, HSIL remains different.
Many arguments are advanced for this disparity. First, HRA is not well reimbursed and we presently only have a T, or trial code. Second, many surgeons report it is a painful procedure [20]. Although the latter may be valid, surgeons routinely perform hemorrhoidectomies, a procedure with a very similar, if not worse, postoperative pain profile. Third, many cite a lack of data to support that HRA can prevent cancer or that HSIL actually progresses to cancer. Papers from our institution and others refute this claim [9, 12, 13]. Additionally, the same surgeons who demand evidence prior to accepting HRA have adopted new technologies (i.e., PPH) with potentially more severe associated risks and significant complications without clinical trials supporting proven short- or long-term benefit. Finally and most troubling to me is the observation that adoption of laparoscopic surgery, single-incision surgery, robotic surgery, PPH, and other “new” interventions was driven in large part by industry and there is no industry driving HRA. Though this may be simple coincidence, the observation remains somewhat concerning.
HSIL and Anal Cancer: The Problem
Key Concept: Understanding the terminology along with proper documentation of the lesions and their locations is the first step in ensuring we have accurate data on the natural history of HSIL involving the various regions of the perianus.
There is no doubt that anal cancer is increasing in both genders and that it is increasing most rapidly in men [1, 21, 22]. Major challenges facing clinicians treating patients with anal and perianal HSIL and cancer are lack of clear terminology and natural history. The first challenge lies in defining anus and perianus. This issue has been addressed by adopting terminology that all clinicians can agree upon without reference to poorly understood specific landmarks. Thus, an anal cancer is defined as a squamous cell carcinoma that may not be seen at all or in its entirety while gentle traction is placed on the buttocks [23]. In contrast, a perianal cancer is a squamous cell carcinoma within 5 cm of the anus that is completely visualized while gentle traction is placed on the buttocks (Fig. 22.2). The transformation zone was introduced because many clinicians were confused by squamous cell carcinomas occurring in the distal rectal mucosa. The transformation zone is a fluid region of squamous metaplasia occurring 0–10 cm proximal to the dentate line where squamous metaplasia is commonly found. The metaplastic tissue is at particular risk for HPV infection.
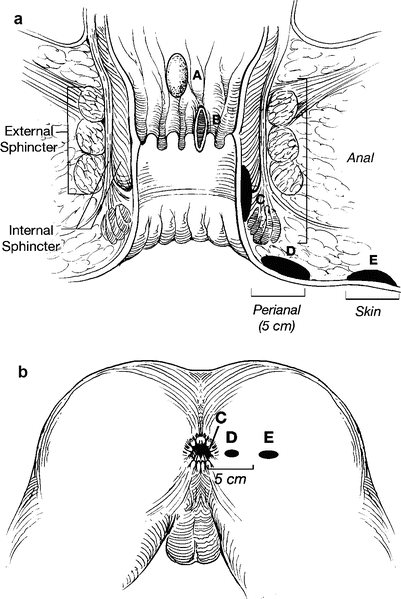

Fig. 22.2
(a, b) Classification scheme for defining lesions as anal or perianal that does not rely on relationship to dentate line. Tumors A, B, and C represent anal lesions that are not visible or are incompletely visible while gentle traction is placed on the buttocks. Tumor D is a perianal tumor because it is completely visible with gentle traction on the buttocks, and lesion E is a skin cancer (With permission from Welton and Raju [45])
A first step in understanding the natural history of anal and perianal cancers, and their precursor lesions, is an accurate reporting of exactly where the lesion was found. The current literature often confuses the two regions, leading to uncertainty as to how best to approach premalignant lesions in either zone. This confusion is evident while trying to reconcile the recommendations to treat Bowen’s disease and observe HSIL [10, 24, 25]. Why would the recommendation be to treat patients with “Bowen’s disease” with a highly morbid procedure – punch biopsies, frozen section-directed wide local excision, and flap reconstruction with proximal diversion if the recommendation was to observe HSIL of the distal rectal mucosa (Fig. 22.3)? We currently lack any data to support they are biologically different [26]. If the argument is that because of the field defect, we don’t know which lesion will progress, then observation of a perianal skin lesion is much less risky and morbid as small perianal cancers can be easily identified and locally excised. In contrast, squamous cell carcinomas arising in the distal rectal mucosa are difficult to identify early, and all but the smallest and most superficial are treated with chemoradiation therapy. Similarly the argument to observe distal rectal and anal mucosal lesions because “the natural history of HSIL” is unclear fails to hold up to scrutiny. The natural history of perianal “Bowen’s disease” is also unclear. Even with wide local excision, patients develop recurrent HSIL and cancer. This disparity in treatment recommendations for similar disease processes highlights our lack of understanding. Due to the confusion around histologic terminology for lower anogenital intraepithelial lesions involving the cervix, vagina, vulva, penis, scrotum, anus, and perianal skin, the Lower Anogenital Standard Terminology conference was held in March of 2012. This was a large multidisciplinary conference involving pathologists, gynecologists, infectious disease specialists, urologists, colorectal surgeons, and others in an effort to clarify and standardize terminology. The results of the consensus conference were published in June 2012 [3] and the recommendations for anal intraepithelial neoplasia were:
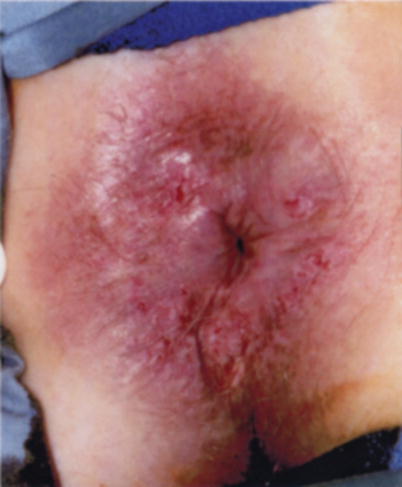

Fig. 22.3
Perianal HSIL (previously known as Bowen’s disease) (With permission from Welton and Raju [45])
1.
Standardize terminology using the above anatomic definitions.
2.
Standardize pathology reporting into two categories low-grade squamous intraepithelial lesion (LSIL) and high-grade squamous intraepithelial lesion (HSIL) with modifiers of AIN II and III as needed.
3.
Report lesions as microinvasive if ≤ 3 mm of invasion seen on histologic evaluation of completely excised lesions that are less than 7 mm in size.
4.
Consider local excision adequate treatment of microinvasive lesions.
5.
Consider p16 IHC evaluation to distinguish precancer from precancer mimics such as immature squamous metaplasia.
By using standardized terms that refer to standardized pathologic findings of tissues taken from known locations, we may develop a more robust understanding of which lesions of the anus and perianus will progress to cancer. Currently, the lack of a clear understanding of the natural history of HSIL is erroneously used as an argument for nontreatment [24, 25]. On the contrary, the natural history of untreated Bowen’s disease is also unclear, and yet radical excision has been the standard treatment for a long time. Yet, despite this aggressive approach, some lesions still progress to cancer [10], just as some untreated HSIL will progress to cancer [9, 24, 25]. In a recent review, it was noted that progression rates appear to be lower in the anus than in the cervix [27]. Yet in the cervix, the natural history data looks at progression rates over 30 years, something we don’t have for lesions of the perianus. Further, the natural history of untreated HSIL of the cervix and vulva suggests 30–50 % will progress to cancer over 30 years [28]. Looked at the other way, this means that 50–70 % won’t progress to cancer over thirty years. As in the anus and perianus, it is undefined which gynecologic lesions will progress (and when), and yet there is uniform agreement to treat HSIL of the cervix, vagina, and vulva. I am not arguing that we shouldn’t treat HSIL of the cervix. The huge drop in cervical cancers after the advent of cervical Pap smears and directed ablation is a modern medical success story. I am simply arguing for consistency in medical practice. Unfortunately, we now have clear documentation untreated anal HSIL progressing to cancer vacating the lack of data and progression argument [13].
Finally, if we can eventually distinguish anal and perianal cancers from premalignant lesions and each other, we may be able to address a suggestion from the gynecological literature regarding a possible difference between cervical and vulvar biology. In this paradigm, just as vulvar lesions are less aggressive than cervical, perianal squamous cell carcinomas may prove to have a less aggressive biology than those of the anal canal [21]. If this held true, perhaps this could lead to varying treatment recommendations based on the lesion location and inherent differences in rates of progression among them. Unfortunately, whether these differences exist, and if they do, whether they are due to differences in blood supply, lymphatic drainage, exposure to trauma, or immunologic differences in different types of squamous epithelia need to be more clearly elucidated. While we do know that risk factors for developing anal HSIL include HIV, HPV, anoreceptive intercourse, more than five anal sex partners, use of illicit drugs, older age at first anoreceptive act, infection with high number of HPV types, smoking, multiple partners, and a history of cervical dysplasia or cervical cancer, we lack accurate information on how these factors meld together to lead some lesions progressing while others do not [21, 29]. Furthermore, we don’t know exactly why someone with no risk factors or high-risk behaviors may present with aggressive disease. Hopefully, going forward, more accurate and uniform documentation will result in answers to these questions.
Treatment
Key Concept: You cannot apply one set of rules uniformly across all risks categories. While you should follow general principles, treat each patient individually taking into account their risk profile, disease burden, treatment history, and your level of concern with the lesions present.
For those not familiar with managing this disease, it should be stated that treatment of anal and perianal HSIL is not technically challenging. In fact, often the only technically challenging aspect of the treatment is adequate visualization of the lesions. This skill set has been mastered by gynecologists, family practitioners, internist, oncologists, nurse practitioners, and some general and colorectal surgeons, and you can master it as well. Courses in how to perform HRA are taught at least twice each year by the American Society for Colposcopy and Cervical Pathology (ASCCP).
High-Resolution Anoscopy (HRA): Initial Examination and Technique
Briefly, HRA involves the magnified visualization of the distal rectal mucosa, anal mucosa, and perianal skin through an operative microscope or colposcope after pretreatment with 3 % acetic acid. HSIL will stand out against the background of acetowhitening as a distinct vascular pattern within the acetowhitened mucosa or skin. The vessel changes are characteristic of HSIL regardless of underlying tissue type – cervix, distal rectal mucosa, or anus (Fig. 22.4). These lesions are biopsied as needed and targeted for focal destruction while sparing the surrounding normal mucosa. In contrast to popular belief, when lesions in the insensate distal rectal mucosa are treated, the patient may experience essentially no postoperative pain. Treated lesions of the anus and perianus that involve the sensate anal mucosa and perianal skin result in postoperative pain similar to hemorrhoidectomies and other benign anorectal conditions that colorectal surgeons commonly treat.
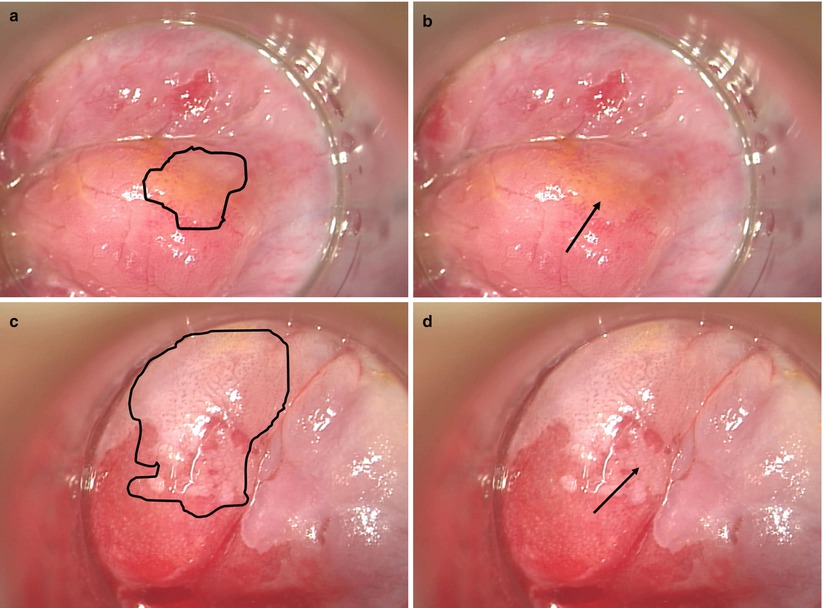

Fig. 22.4
High-resolution anoscopy images of LSIL and HSIL after the application of acetic acid. Biopsies of visualized lesions confirmed HRA appearances, and region biopsied is indicated with arrows in images (b) and (d). Panels (a) and (b) demonstrate anal LSIL in the distal rectal mucosa with subtle punctate vessel changes. The geography of the lesion is emphasized in the left frame with a black border. (c, d) Distal rectal mucosa where HSIL is visible. The left image has the lesion highlighted with a black border focusing the reader on the serpiginous, cerebriform vessels and the outline of the entire lesion. The right image demonstrates the mosaic pattern created by blood vessels in an acetowhite background (With permission from Welton and Raju [45])
I prefer the operating room for the initial examination and treatment as well as for re-treatment of extensive disease, disease overlying engorged hemorrhoidal cushions, and for disease complicating, or complicated by, other benign anorectal diseases [30]. The patient is treated in the prone jackknife position with the buttocks taped apart. Anesthesia is MAC local with 0.25 % Marcaine in the subcutaneous tissue and 0.5 % Marcaine in the sphincters for an anal block. This allows for excellent visualization of most lesions. A thorough digital rectal examination is carried out focusing on subtle changes in the skin, mucosa, and submucosa of the perianus and distal rectum. This initial exam often focuses my subsequent visual inspection. I examine the perianal skin looking for any hyperpigmentation, erythema, elevation, or scaling consistent with a lesion. I think conduct a routine anoscopy with a Hill Ferguson anoscope, visualizing the distal rectal mucosa and anal mucosa. Next, I place one acetic acid soaked raytec in the anal canal and distal rectum. I place another one over the anus and perianus. I position the operative microscope over the anus and begin with a thorough evaluation of the perianal skin, noting location of worrisome lesion for subsequent biopsy and destruction. I then thoroughly evaluate the anal mucosa and distal rectal mucosa in a circumferential fashion taking care to visualize any abnormalities palpated on digital rectal examination. All lesions concerning for HSIL are biopsied treated with needle tip electrocautery taking care to avoid burning deeply by moving the cautery tip quickly smoothly across the surface of the lesion. If the underlying hemorrhoidal tissues are disrupted, this is generally controlled without difficulty using cautery alone. Very rarely I have had to control hemorrhoidal hemorrhage with a chromic catgut ligature placed at the apex of a hemorrhoidal cushion. Overall, I prefer the operating room, at least initially, to office-based therapies as I feel this allows for better visualization and determination of extent of disease. I have often found disease along the hemorrhoidal column that would not have been identified without the relaxation and visualization provided by MAC local. The relaxation of the sphincters allows for flattening of the distal rectal mucosa and improved lesion detection. Without this improved visualization, I believe lesions are missed and this results in the false impression that lesions have “returned” when in fact the lesion is persistent and was never adequately addressed in the first place.
Dealing with Recurrence
When lesions do recur, they may be treated in the office with trichloroacetic acid or infrared coagulation (IRC). As noted above, some recurrences are best treated in the operating room. More importantly, we, and others, have experienced excellent control of HSIL and minimal progression to cancer with this approach [6, 8, 9, 12].
Issues around HRA and cautery destruction that have discouraged more widespread adoption are the incorrect belief that all lesions recur (so why treat them in the first place and risk complications like nonhealing wounds or stenosis) and the patients experience significant pain (see above for this myth buster). Furthermore, reimbursement is poor, especially given the time and effort required to develop and hone the new skill set necessary to visualize and treat these lesions. Our group and others have shown that despite initial recurrences, HSIL can be cleared in ~80 % of patients and progression to cancer can be significantly diminished (Pineda and Goldstone) [6, 8, 9, 12, 20]. This is done through targeted destruction in the operating room with follow-up destruction in clinic as needed. Admittedly, the patients do experience postoperative pain. Yet, this can be largely controlled with sitz baths (in a bathtub filled to the chest), LMX-5 % topical lidocaine cream, and a narcotic agent. I have found that the warm bath and LMX-5 % are the most effective methods of pain relief.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








