Severity scores for alcoholic hepatitis
Alcoholic hepatitis (AH) is a distinct subset of patients with alcoholic liver disease and has a potential for high mortality within 3 to 6 months after clinical presentation. Mild forms of AH usually improve with conservative management. However, patients with severe AH have been reported to have 30-day mortality of up to 50%. Therefore, assessment of the disease severity becomes an important and practical issue for clinicians involved in the management of patients with AH. Many scoring systems have been developed for use in clinical practice.
Discriminant Function Index
The discriminant function index (DFI) was initially described by Maddrey and colleagues in a placebo-controlled study to assess the benefit of corticosteroid (CS) therapy in 55 patients with AH. Using the formula: 4.6 × prothrombin time (PT) in seconds + serum bilirubin (mg/dL), patients with a DFI above 93 and treated with placebo had a 28-day survival of 25%, whereas those with a score of 93 or lower had 100% survival. In 1989, this score was modified (modified discriminant function or mDF) using prolongation of PT in seconds (over control) instead of absolute value of PT ( Table 1 ). Patients without treatment and mDF score of 32 or higher and/or the presence of encephalopathy had a 28-day survival of about 65%. A recent analysis confirmed this observation with untreated patients having 28-day survival of 68% among patients with mDF of 32 or higher. The American College of Gastroenterology recommends that AH patients with mDF score of 32 or higher should be considered for CS therapy.
| Scoring System | Parameters | Formula | Severe Disease | |||
|---|---|---|---|---|---|---|
| DFI | SB and PT | 4.6 × (patient’s PT – control PT in seconds) + SB | ≥32 | |||
| CPT | SB, PT, serum albumin, ascites, and PSE | 1 | 2 | 3 | ≥7 | |
| SB | <2 | 2–3 | >3 | |||
| PT↑ | <4 | 4–6 | >6 | |||
| Albumin | >3.5 | 2.8–3.5 | <2.8 | |||
| Ascites | Absent | Slight | Tense | |||
| PSE | None | Grade I–II | Grade III–IV | |||
| MELD score | Age, SB, INR, and SC | 9.57 log e (SC) + 3.78 log e (SB) + 11.2 log e (INR) + 6.43 Available at: www.mayoclinic.org/meld/mayomodel7.html | ≥21 | |||
| GAHS | Age, BUN, WBC, SB, and INR | 1 | 2 | 3 | ≥9 | |
| Age | <50 | ≥50 | — | |||
| WBC | <15 | ≥15 | — | |||
| BUN | <14 | ≥14 | — | |||
| SB | <7.3 | 7.3–14.6 | >14.6 | |||
| Tc>INR | <1.5 | 1.5–2.0 | >2.0 | |||
| Lille score | Age, labs at day 0 (SB, albumin, and PT), and change in SB at day 7 | 3.19–0.101 × age in yrs + 0.147 × albumin (g/L) on day 0 +0.0165 × change in SB (μmol/L) – 0.206 × RI (0 if absent and 1 if present) −0.0065 × SB on day 0 (μmol/L) −0.0096 × PT (in seconds) | ≥0.45 | |||
| ABIC score | Age, bilirubin, INR, and creatinine | (age × 0.1) + (SB × 0.08) + (SC × 0.3) + (INR × 0.8) | ≥9 | |||
The advantages of the mDF are its simplicity of calculation and validation in many clinical trials. However, nonstandardization of the PT testing with laboratory to laboratory variation depending on the type of thromboplastin used by the laboratory is a limitation. Because patients with a DFI greater than 32 and an absence of encephalopathy are not considered for specific treatment, the mDF should have an accuracy of close to 100% in predicting their survival. However, patients with an mDF of less than 32 may have a 28-day mortality rates of about 7% to 17%. Overall sensitivity and specificity of predicting mortality in 1 study was 67% and 62%, respectively. One of the ways to tackle this issue is to lower the threshold score for initiating treatment. However, the risk benefit ratio does not favor CS treatment of patients with mDF score of less than 32.
Child–Turcotte–Pugh Score
This scoring system is based on 3 objective (serum bilirubin, serum albumin, and PT) and 2 subjective (hepatic encephalopathy and ascites) variables and categorize into 3 stages (A–C) with a total score of 5 to 6, 7 to 9, and greater than 9, respectively. The score is traditionally used for cirrhotic patients with mortality rates of about 10% to 15%, 25% to 30%, and 70% to 80% at 1 year for stages A, B, and C, respectively. Although not a traditional scoring system for AH patients, the Child–Turcot–Pugh (CTP) score was useful in predicting mortality at 3 to 6 months (see Table 1 ). Presently, the CTP score is not widely used for assessing severity of AH.
Model for End-Stage Liver Disease Score
Model for End-Stage Disease (MELD) score is being widely used for prediction of mortality in end-stage liver disease and is universally used to prioritize patients for liver transplantation (LT). Some studies have examined the use of MELD in assessing severity of AH. In a study on 34 patients with AH, the area under receiver operating characteristic curve (AUROC) for the MELD score was 0.82 with sensitivity and specificity in predicting 30-day mortality for a MELD score above 11 being 86% and 81%, respectively. In another study on 73 patients with AH, MELD score of 21 had highest sensitivity and specificity to predict mortality at 30 and 90 days. In yet another study on 202 patients, a first week MELD score of 20 or greater and an increase of MELD score at 1 week of 2 or more points had the highest sensitivity and specificity in predicting mortality. Recently, MELD-Na has been shown to be superior than MELD score among AH patients and ascites.
The advantage of MELD score is the use of the International Normalized Ratio (INR) (instead of PT). This is more comparable across laboratories, because the calculation accounts for the sensitivity of the thromboplastin reagent used in the test. However, the formula for calculating MELD is complex; however, this can be easily overcome using a computer program or calculating the MELD score online (see Table 1 ). Another problem with the use of MELD score is variation across studies in the best cutoff score in predicting mortality from 11, 18, to 21 to 22. Much of the variability, though, is due to use of original versus newer iterations of the MELD score that assign points differently. American Association for Study of Liver Diseases guidelines on the management of acute liver disease suggest that a cutoff MELD score of 18 be taken to predict severe AH and be the criterion for initiating treatment.
Glasgow Alcoholic Hepatitis Score
The Glasgow alcoholic hepatitis score (GAHS) was introduced in 2005 to assess AH severity (see Table 1 ). The score ranges between 5 and 12; a GAHS of 9 or higher at days 1 and 7 was more accurate than the mDF in predicting survival at 28 days ( P = .0016 and P = .0038, respectively) and at 84 days ( P = .0179 and P = .0477, respectively). GAHS at day 7 but not at day 1 was better in predicting 28-day outcome than the MELD score ( P = .0339 and P = .069). GAHS at days 1 and 7 was more accurate than the MELD score in predicting the 84-day outcome ( P = .0005 for both scores). GAHS was reliable in predicting mortality irrespective of the use of INR or PT for determination of coagulation status or use of liver biopsy for diagnosis of AH. GAHS was confirmed in a validation cohort of 195 patients in this study; however, the score has not been validated outside the United Kingdom.
Recently, the GAHS has been shown to predict response to CS treatment. In this retrospective study, 144 AH patients with an mDF of 32 or higher were included. Of this group, 73 (51%) with a GAHS of 9 or greater were treated with CS. Patients with a GAHS below 9 did not differ on 28- and 84-day survival whether or not treated with CS (84% vs 80% and 73% vs 68%, respectively). However, the survival of patients with a GAHS of 9 or greater was better with CS treatment at day 28 (78% vs 52%; P = .002) and at day 84 (59% vs 38%; P = .02).
Age–Bilirubin–INR–Creatinine Score
A new score, the age–bilirubin–INR–creatinine (ABIC) score, has been suggested for prognostic stratification of patients with AH (see Table 1 ). Using the cutoff values of 6.71 and 9.0, authors identified patients with low, intermediate, and high risk of death at 90 days (100%, 70%, and 25% of survival rate, respectively; P <.0001). Using the same cutoff values, the ABIC score also stratified patients according to their risk of death at 1 year. The AUROC for predicting mortality at 90 days using ABIC score was 0.82 (95% confidence interval [CI], 0.73–0.91; P = .0001). In comparison, AUROC values were lower for the mDF score, MELD score, and GAHS: 0.70 (95% CI, 0.56–0.84; P = .008), 0.76 (95% CI, 0.64–0.88; P = .0004), and 0.75 (95% CI, 0.63–0.86; P = .001), respectively. On multivariate analysis, ABIC score was the best independent predictor of 90-day mortality (hazard ratio, 2.78; 95% CI, 1.90–4.09; P = .0001).
Early Change in Bilirubin Level
In a retrospective study on 238 biopsy-proven AH patients, a decrease in serum bilirubin at 1 week or early change in bilirubin level (ECBL) was a predictor for survival. A total of 73% patients showed ECBL at 1 week (4.9–4.4) and 83% of them survived. In contrast, the remaining cases did not show ECBL, with only 23% survival. In another study reported from France assessing role of N -acetylcysteine (NAC) in combination with CS, decrease in bilirubin at day 14 was a significant predictor of survival.
Lille Model
About 40% of patients with severe AH fail to respond to treatment with steroids. In a prospective study on 320 biopsy-proven severe AH, nonresponders to steroids (NRS) could be identified based on ECBL and other five variables. This led to the development of Lille score (see Table 1 ).
Survival at 6 months was lower for patients with a Lille score of 0.45 or higher compared with patients with Lille score of less than 0.45 (25% vs 85%; P <.0001). The AUROC value for the Lille score cutoff of 0.45 was higher than the CTP score (0.89 vs 0.62; P <.00001) or mDF score (0.89 vs 0.66; P <.00001). The authors concluded that CS be discontinued for patients with a Lille score of 0.45 or greater at 1 week. The Lille score maintains accuracy in predicting the survival when used across a range. In a retrospective study on 641 biopsy-proven AH, a linear correlation with survival was seen among groups with Lille score of less than 0.16, 0.16 to 0.56, or greater than 0.56 with survival rates of 87%, 70%, and 21%, respectively, at 6 months. Although this score has not been validated outside France, the score has been validated prospectively in other studies from the same center. Unfortunately, the score does not guide initiation of treatment because it cannot be calculated at admission.
Comparison of Scores
Many studies have compared available scoring systems assessing severity of AH. MELD and DFI have been compared among 6 studies. The data have shown differences across studies ( Table 2 ).
| Author (Year) | Country | Comparison of | Type of Study | n | Diagnosis of AH | Outcome | Score Cutoff | Findings |
|---|---|---|---|---|---|---|---|---|
| Sheth et al (2002) | USA | MELD and DFI | Retrospective | 34 | Clinical | 30-day mortality | MELD 11 DFI 32 | MELD: Sensitivity 82%, specificity 82%, AUROC 0.82 DFI: Sensitivity 86%, specificity 48%, AUROC 0.86 |
| Said et al (2004) | USA | MELD and CTP | Retrospective | 98 a | Clinical | 3- and 6-month mortality | MELD and CTP as continuous scores | 3-month mortality: AUROC MELD vs CTP (0.85 vs 0.85; P = .5) 6-month mortality: AUROC MELD vs CTP (0.83 vs 0.81; P = .33) |
| Dunn et al (2005) | USA | MELD and DFI | Retrospective | 73 | Clinical | 30-day mortality 90-day mortality b | MELD 22 DFI 41 MELD 21 DFI 37 | MELD: Sensitivity 75%, specificity 75%, AUROC 0.83 DFI: Sensitivity 75% specificity 69%, AUROC 0.74 MELD: Sensitivity 75%, specificity 75%, AUROC 0.86 DFI: Sensitivity 88% specificity 65%, AUROC 0.83 |
| Forrest et al (2005) | UK | GAHS and DFI | Retrospective c | 241 | Clinical | 28-day mortality 84-day mortality | GAHS 9 DFI 32 Same | Sensitivity and specificity GAHS vs DFI at admission (54/89 vs 82/39; P = .002) and at day 6-9 (66/85 vs 92/41; P = .04) Sensitivity and specificity GAHS vs DFI at admission (43/90 vs 79/40; P = .018) and at days 6–9 (56/88 vs 88/44; P = .048) |
| Srikureja (2005) | USA | MELD, DFI, and CTP d | Retrospective d | 202 | Clinical | In-hospital mortality | DFI 32 MELD 18 CTP 12 | Sensitivity/specificity/AUROC MELD vs DFI vs CTP at admission (85/84/0.89 vs 83/60/0.81 vs 76/80/0.87; P = NS) At 1 wk AUROC MELD vs DFI: 0.91 vs 0.85; P = .33 and MELD vs CTP 0.91 vs 0.85; P = .35 Change in score at 1 wk AUROC: MELD vs DFI 0.85 vs 0.71; 0.059 and MELD vs CTP 0.85 vs 0.57; P = .0004 |
| Verma (2006) | USA | MELD and DFI | Retrospective e | 99 | Clinical | Septic events, HRS, and short-term mortality | MELD 20 DFI 32 | MELD score and not DFI was independent predictor for outcomes with OR [95% CI] for septic event, HRS, and short-term mortality: 2.8 [1–8; P = .04]; 4.0 [1–17; P = .05]; and 6.4 [1–38; P = .03], respectively |
| Forrest et al (2007) | UK | GAHS and DFI | Retrospective f | 144 | Clinical | 28- and 84-day survival on CS treatment | GAHS 9 DFI 32 | GAHS < 9: No difference in survival among treated and untreated patients at 28 days (84% vs 80%) and 84 days (73% vs 68%) GAHS ≥ 9: Survival better with treatment at 28 days (78% vs 52%; P = .002) and 84 days (59% vs 38%; P = .02). |
| Jeong et al (2007) | Korea | DFI, CPT, and MELD | Retrospective | 74 | Clinical | 90-day mortality | Not available | On multivariate regression analysis, CTP and DFI scores but not MELD could predict the outcome; overall mortality at 90 days was 16% |
| Zapata-Irrison et al (2008) | Mexico | MELD and DFI | Retrospective | 67 | Clinical | Mortality | MELD 21 DFI 32 | Sensitivity/specificity/AUROC of MELD: 96/10/0.73 and of DFI: 100/7/0.69 ( P = NS) |
a Study performed on 1016 patients with liver disease of various causes, including 98 AH patients.
b MELD and DFI similar in predicting mortality; however, on multivariate regression analysis, MELD independently predicted 90-day mortality.
c No patient was treated with CS or pentoxifylline, but antioxidants were allowed. Score validated on a prospective cohort of 195 biopsy-proven AH patients
d Patients with DFI of ≥32 were treated with pentoxifylline and none treated with CS. AUROC for MELD score at admission (cutoff 18), at 1 week (cutoff 20), and change in MELD at 1 week (cutoff 2) was similar.
e Patients with severe AH (DFI ≥ 32) were included. Treatment was with pentoxifylline (as per treating physician discretion) and none of the patients received CS.
f Patients with severe AH (DFI ≥ 32) were included and of this cohort 73% received CS.
Similarly, the MELD and CPT scores have been compared and were found to be similar in predicting survival. Two studies compared MELD, DFI, and CPT scores. Admission scores were similar in predicting mortality; however, MELD was better than other scores when an increase in respective scores was analyzed at 1 week. In contrast, another study found that DFI and CPT but not MELD scores independently predicted survival.
Two studies from the same center compared GAHS and DFI scores. The first study showed that the GAHS is superior to DFI in predicting survival at 28 and 84 days. In another study, the GAHS was shown to be superior to DFI for predicting the response to CS treatment.
The retrospective design of these studies, small sample size, and heterogeneity of the sample population are some of the potential reasons for the conflicting observations among various studies. Some of the reasons for heterogeneous population are variations in (1) proportion of use of liver biopsy in diagnosis of AH, (2) proportion of patients having concomitant cirrhosis, (3) inclusion and exclusion criteria, (4) proportion of patients receiving specific treatment, and (5) time at which survival is estimated. A recent study overcoming some of these limitations has compared the ABIC, GAHS, DFI, MELD, and DFI scores in a prospective cohort of 332 biopsy-proven AH patients. The Lille score was found to be superior to all other scores with an AUROC of 0.84. However, the MELD and ABIC scores were also useful with AUROC of 0.7 or higher.
An ideal score should be simple, accurate, credible, objective, and validated in a prospective cohort both within and outside the country of origin. Furthermore, the score should be able to guide treatment initiation and response. However, we still do not have a single scoring system that passes all these criteria ( Table 3 ). Until the search for an ideal scoring system is completed, the mDF score will continue to be used for initiating treatment and the Lille score for guiding treatment response. The MELD and GAHS scores are also currently useful for initiating treatment with cutoffs at 18 and 9, respectively. One novel area of investigation would be to examine whether a combination of existing scoring systems can meet the criteria for an ideal score for assessing AH patients.
| Simplicity | Accuracy | Credibility | Objectivity | Validity | International Validation | Direction for Treatment | Treatment Response | |
|---|---|---|---|---|---|---|---|---|
| CTP score | ++ | + | + | +/− | ++ | ++ | − | − |
| mDF score | ++ | ++ | ++ | ++ | ++ | ++ | ++ | +/− |
| MELD score | +/− | ++ | ++ | ++ | ++ | ++ | +/− | +/− |
| GAHS | ++ | + | + | ++ | ++ | −− | +/− | + |
| Lille score | +/− | ++ | ++ | ++ | ++ | −− | −− | ++ |
| ABIC score | +/− | + | + | ++ | + | − | +/− | −− |
Treatment of AH
Abstinence From Alcohol
Abstinence is of paramount importance in the treatment of AH and has been shown to significantly improve long-term survival. The success rate of achieving abstinence varies across different studies using different treatment approaches for achieving alcohol abstinence. Factors associated with long-term abstinence are patient’s awareness of the consequences of alcohol consumption, adequate social support, lack of illicit drug use, and absence of psychiatric comorbidities. Incorporation of behavior modification and Alcoholics Anonymous increases the abstinence rates and is recommended for patients who have difficulty in abstaining.
Pharmacologic therapy such as naltrexone (an opioid receptor antagonist), acamprosate (GABA analog), and baclofen (GABA agonist) can be used to maintain abstinence. However, only baclofen has been tried in patients with cirrhosis and liver failure. In this study, patients with alcoholic decompensated cirrhosis were randomized to receive baclofen (n = 42) or placebo (n = 42). At 3 months, abstinence rates were better with use of baclofen compared with placebo-treated patients (71% vs 29%; P = .0001) with longer cumulative abstinence duration (63 vs 31 days; P = .001). No side effects were reported with the use of baclofen in these patients with advanced cirrhosis.
Management of Alcohol Withdrawal
In patients with a history of alcohol abuse, it is crucial to recognize symptoms of alcohol withdrawal, including insomnia, irritability, nausea, vomiting, headache, anxiety, cardiac arrhythmia, hypoglycemia, and diaphoresis. Rarely, withdrawal tonic clonic seizures may occur which can proceed to delirium tremens (DT). DT defined by hallucinations, disorientation, cardiac arrhythmia, hypertension, fever, agitation, and diaphoresis, usually occurs 48 to 96 hours after the patient’s last alcohol drink. Risk factors for the development of DT include chronic alcohol use, history of DT in the past, elevated serum alcohol levels, and presence of concomitant illness. Mortality rate of DT approaches 5% and is usually owing to arrhythmia and complicating illness (pancreatitis, hepatitis, or pneumonia). Benzodiazepines are used for prophylaxis and acute withdrawal with lorazepam and oxazepam being the preferred agents because of their relatively short half-lives. Refractory DT may be treated with the addition of phenobarbital to benzodiazepine therapy. Propofol has also been utilized to control symptoms. Patients who require phenobarbital or propofol will likely need endotracheal intubation and mechanical ventilation.
Treatment of Complications of Chronic Liver Disease
About 40% to 50% of patients with AH may have underlying cirrhosis. Therefore, these patients may frequently have complications of cirrhosis such as ascites, infection particularly spontaneous bacterial peritonitis, variceal bleeding, altered mental status, and renal insufficiency with hepatorenal syndrome (HRS). The management of these complications is similar to any other patient with cirrhosis. This will not be detailed here and only a brief outline is provided ( Table 4 ).
| Complication | Initial Evaluation | Treatment Measures | Treatment if Refractory to Routine Measures |
|---|---|---|---|
| Variceal bleeding | CBC, LFTs, BMP | Octreotide infusion, antibiotics, EGD and EVL | TIPS; Shunt surgery for compensated cirrhosis |
| Ascites | LFTs, ascetic fluid analysis | Diuretics, IV albumin, paracentesis | TIPS |
| Spontaneous bacterial peritonitis | Ascitic fluid analysis with gram stain and C/S, blood C/S | Antibiotics, IV albumin | Change antibiotics based on C/S |
| Hepatic encephalopathy | Detailed history and evaluation to identify precipitant | Treat the precipitant factor, lactulose | Rifaximin |
| Hepatorenal syndrome | LFTs, BUN, SC, UA, infection work-up, fluid challenge and discontinue diuretics | Midodrine, octreotide, terlipressin | TIPS |
Malnutrition
Patients with AH frequently present with protein caloric malnutrition due to a number of factors such as poor intake, decreased small intestinal absorption, and alcoholic diarrhea. Maintaining an adequate nutritional status is crucial because the outcome is directly related to the nutritional status of these patients. Physical examination and laboratory evaluation should be performed for mid-arm circumference, triceps skin-fold thickness, creatinine height index, total lymphocyte count, and prealbumin levels to provide a comprehensive nutritional status of the patient. Patients who are found to be malnourished should specially be targeted for providing rigorous nutritional support.
Many studies have assessed the role of nutritional supplementation in the treatment of AH. A randomized controlled trial (RCT) compared CS therapy (40 mg/d) with enteral diet (2000 kcal/d). Overall mortality in both groups was similar. However, the enteral nutrition group had a lower incidence of infections. In another RCT, 263 patients with moderate to severe AH were randomized to receive prednisolone, oxandrolone, or placebo. Patients receiving oxandrolone and nutritional supplementation had a better outcome as opposed to oxandrolone alone.
It is important to achieve nutritional goals with positive nitrogen balance to improve survival, therefore an energy intake of 35 to 40 kcal/kg per day and protein intake of 1.2 to 1.5 g/kg per day is recommended. Proteins should not be restricted even in patients with encephalopathy, provided they can tolerate the protein load and encephalopathy does not worsen. The use of a daily caloric count and the help of a professional dietitian are crucial to identify patients who need additional supplementation. Enteral supplementation is preferred and enteral tubes can be safely placed even in patients with unbanded esophageal varices.
Specific Pharmacologic Agents for the Treatment of AH
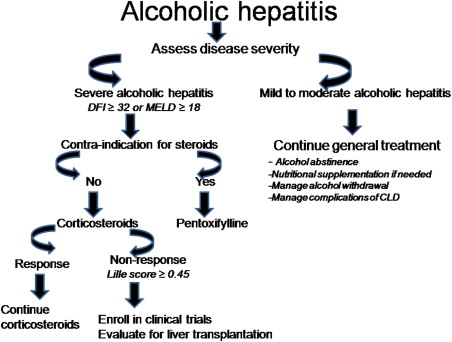
Because patients with severe AH have a mortality rate of about 40% to 50% in first 6 months, they should be treated with specific pharmacologic agents. Many agents have been tried ( Fig. 1 ) and CS use is recommended by some as the initial drug of choice.
Corticosteroids
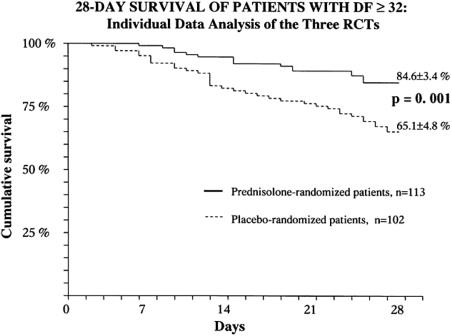
Over the last about 3 decades, 12 placebo-controlled RCTs have been performed to assess benefit of CS in AH ( Table 5 ). Data are conflicting with only 5 studies showing a survival benefit (see Table 5 ). Variations in sample size, inclusion/exclusion criteria especially on the use of liver biopsy for diagnosis, disease severity, endpoints, type of CS used, and treatment duration could explain the differences among studies. Meta-analyses on the efficacy of steroids in AH have also shown conflicting data. However, when the individual patient data on 132 patients with severe AH (mDF ≥32) from 3 RCTs were pooled, CS were found to be beneficial in improving survival at 28 days as compared with placebo (85% vs 65%; P = .001; Fig. 2 ). The benefits of CS for patients with severe AH were also confirmed in a Cochrane analysis despite the heterogeneous data.
| Author/Year | Sample Size | Mean Age (y) | Males (%) | Drug Schedule | Time to Survival | Survival (Treated vs Placebo) | Secondary Findings |
|---|---|---|---|---|---|---|---|
| Helman et al (USA) 1971 e | 37 (20) | 48 | 32 | Prednisolone 40 mg/d × 4 wks | 28 days | Benefit only for severe AH (93% vs 60%; P <.01). | No difference on histology at 4 wks and no effect on prevention to cirrhosis. |
| Porter et al (USA) 1971 | 20 (11) | 45 | 64 | 6-MP 40 mg/d × 10 d | In hospital | 45% vs 22%; P = NS | No effect on biochemical parameters |
| Campra et al (USA) 1973 c | 45 (20) | 43 | 75 | prednisone 0.5 mg/k/d × 3wks then 0.25 mg/k/d × 3 wks | 6 weeks | 36% vs 35%; P = NS | No effect on biochemical parameters and trend for improved survival with HE ( P = .2) |
| Blitzer et al (USA) 1973 | 28 (16) | 48 | NR | Prednisolone 40 mg × 14 days then tapering × 2 wks | In hospital | 50% vs 69%; P = NS | No effect on biochemical parameters. |
| Shumaker et al (USA) 1978 | 27 (12) | 44 | 75 | 6-methyl prednisolone 80 mg/d × 4–7 d PO | In hospital | 50% vs 53%; P = NS | Patients with C/I to steroids had higher mortality. Causes of death in the 2 groups were similar with >50% dying from GIB |
| Maddrey et al (USA) 1978 | 55 | 40 | 60 | prednisolone 40 mg/d × 28–32 days | 30 days | 96% vs 80%; P = .1 | SB > 20, PT > 8 sec prolonged and encephalopathy predicted mortality. No effect on development of portal hypertension. |
| Lesesne et al (USA) 1978 a | 14 (7) | 49 | NR | Prednisolone 40 mg/d ×30 d then 2 wks of tapering | NR | 71% vs 0%; P <.01 | Infrequent complications from steroids could be cause of death. |
| Depew et al (USA) 1980 9 | 28 (15) | 49 | 66 | Prednisolone 40 mg/d × 28 d then taper × 14 d | 60 days | 53% vs 54%; P = NS | No effect on biochemical parameters and complications higher with steroids. |
| Theodossi et al (UK) 1982 c | 55 (27) | NR | 70 | Methylprednisolone 1 g/d × 3d | 28 days | 37% vs 43%; P = NS | Survival predicted by: HE, DF >93, bilirubin 20 mg/dL, creatinine 3 mg/dL, and histologic evidence of cirrhosis. |
| Mendenhall et al (USA) 1984 b | 263 (90) | 51 | 100 | Prednisone tapered over 28 days | 30 days | Survival similar | Overall mortality 13% for moderate and 29% for severe AH. Oxandrolone improved long-term survival. |
| Carithers et al (USA) 1989 d | 66 (35) | 43 | 62 | Methylprednisolone 32 mg/d for 28 days then tapered over 2 weeks | 28 days | 94% vs 65%; P = .0006 | Survival with HE: 93% vs 53%; P = .02 |
| Ramond et al (USA) 1992 d , e | 61 (32) | 48 | NR | Prednisolone 40 mg/d × 28 d [IV if unable to take PO] | 66 days | 84% vs 45%; P = .002 irrespective of HE (21/23 vs 10/19; P <.001). | Death in steroids group occurred early. |
a Includes patients with hepatic encephalopathy and compared prednisolone to caloric supplements with 1600 kcal/d.
b One hundred thirty-two patients had moderate and 131 severe AH; 85 received oxandrolone and 88 received placebo.
c Included severe AH patients defined based on clinical grounds or serum bilirubin > 5 mg/dL, or hepatic encephalopathy.
d Included severe AH patients defined by mDF ≥ 32 and/or hepatic encephalopathy.
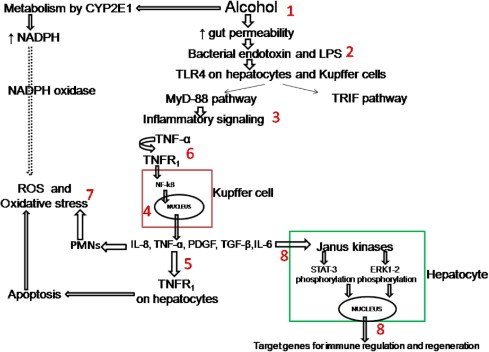
CS act by reducing inflammatory cytokines such as tumor necrosis factor (TNF)-α, intercellular adhesion molecule-1, interleukin (IL)-6, and IL-8. A decrease in the cytokines and other inflammatory markers was shown in a randomized study among patients given CS for 8 days as compared with patients receiving placebo. Peripheral white blood cell count above 5500/mm 3 and amount of polymorphonuclear leukocytic infiltration on the liver biopsy specimen have been shown to predict response and survival on CS treatment. Recently, insensitivity of lymphocytes to steroids has been shown in patients with AH, which improves on recovery among those who respond to steroids. In the same study, ex vivo use of theophylline reversed the lymphocyte insensitivity to CS, suggesting a possible adjunctive role of theophylline in the treatment of AH.
Although many agents have been used across different studies, prednisolone is preferred (but not demonstrated to be better) over prednisone because prednisone requires conversion within the liver to its active form, prednisolone. The drug is given orally in a dose of 40 to 60 mg/d for a total duration of 4 weeks. The treatment is then tapered over next 2 to 3 weeks. If the patient is unable to take orally owing to nausea, vomiting, or altered sensorium, an intravenous preparation such as methylprednisolone may be used until the patient is capable to take medication by mouth.
It is prudent to screen patients for any contraindication before starting steroids. One of the most important contraindications is the presence of infection, which is fairly common among patients with severe AH. This used to be considered an absolute contraindication for steroids. However, if patients are adequately treated for an established infection, CS can be safely administered and improve the outcome. In a prospective study on 246 severe AH patients, about 25% had infection requiring antibiotic treatment before starting CS. Survival at 2 months in this group was similar to patients who were not infected (71% vs 72%; P = .99). Other contraindications are an active gastrointestinal bleeding, renal failure, acute pancreatitis, active tuberculosis, uncontrolled diabetes, and psychosis.
Patients who achieve decrease in bilirubin at 1 week of starting CS have a better survival at 6 months compared with those whose bilirubin does not change or increases (83% vs 23%; P <.0001). Patients with a Lille score of 0.45 or higher are defined as NRS and this is accurate in predicting 75% of deaths at 3 to 6 months. Patients with NRS at 1 week do not benefit from continuing CS and are at risk for infection and sepsis.
Pentoxifylline
Pentoxifylline (PTX), a TNF-α inhibitor and nonspecific phosphodiesterase inhibitor is an alternative option for patients with AH ( Fig. 3 ). Based on the pilot study on the efficacy of PTX in AH patients, a pivotal double-blind, placebo-controlled RCT showed survival benefit at 1 month as compared with placebo (76% vs 54%; P = .037). This benefit was mainly due to the prevention of the HRS among patients treated with PTX (50% vs 92%; P <.05). Later, many studies (reported as abstracts) confirmed this observation of beneficial effect of PTX in the prevention of HRS ( Table 6 ). However, the latest Cochrane systematic review of 5 RCTs (4 reported as abstracts) concluded that there is not enough evidence for survival benefit of PTX in the treatment of AH.
| Author/Year | Sample Size | Mean Age (y) | Males (%) | Drug Schedule | Time to Survival | Survival (Treated vs Placebo) | Secondary Findings |
|---|---|---|---|---|---|---|---|
| McHutchison et al (USA) 1991 a | 22 (12 PTX) | NR | NR | PTX 400 mg TID ×10 d | 30 days | 91% vs 70%; P = NS | Decreased renal dysfunction with PTX. Plasma TNF increased in controls only. |
| Akriviadis et al (USA) 2000 | 101 (49 PTX) | 42 | 71 | PTX 400 mg TID ×28 d | In hospital | 75% vs 54%; P = .037 | Age, creatinine, and PTX treatment predicted survival. TNF levels were no different. However, among nonsurvivors TNF levels decreased more in PTX group. |
| Paladugu et al (India) 2006 a | 30 (14) | 50 | 100 | PTX | 28 days | 71% vs 54%; P = .09. | Time to death 21 vs 18 days (0.041). TNF levels unchanged in both groups. |
| Sidhu et al (India) 2006 a | 50 | NR | NR | PTX 400 mg TID ×28 d | 28 days | 76% vs 60%; P = NS | PTX reduced creatinine, TNF, mDF, PT. |
| Lebrec et al (France) 2007 a | 132 | NR | NR | PTX | 2 and 6 months | 86% vs 84%; P = .77 and 73% vs 69%; P = .3 respectively | Subgroup with renal dysfunction also did not get benefit with PTX. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





