Bladder cancer ranges from a low-grade variant to high-grade disease. Assessment for treatment depends on white light cystoscopy, however because of its limitations there is a need for improved visualization of flat, multifocal, high-grade, and muscle-invasive lesions. Photodynamic diagnosis and narrow-band imaging provide additional contrast enhancement of bladder tumors and have been shown to improve detection rates. Confocal laser endomicroscopy and optical coherence tomography enable real-time, high-resolution, subsurface tissue characterization with spatial resolutions similar to histology. Molecular imaging offers the potential for the combination of optical imaging technologies with cancer-specific molecular agents to improve the specificity of disease detection.
Key points
- •
Improved optical imaging of the bladder can lead to more effective use of bladder-sparing management for low-grade cancer and more aggressive treatment for high-grade cancer.
- •
Photodynamic diagnosis and narrow band imaging are macroscopic imaging modalities and provide contrast enhancement of suspicious lesions that improve detection rates.
- •
Confocal laser endomicroscopy is an example of a microscopic optical biopsy technology that provides high-resolution and subsurface tissue characterization similar to histology.
- •
Confocal laser endomicroscopy is the only clinical technology capable of differentiating high-grade and low-grade bladder cancer.
- •
The highest image resolution is inferred by molecular specificity. The development of molecular markers and binding agents for molecular imaging can serve to differentiate low-grade and high-grade disease.
Introduction
Bladder cancer is the sixth most common cancer in the United States with 74,690 new cases and 15,580 deaths expected in 2014. The natural history of bladder cancer is heterogeneous, ranging from low-grade variant that does not recur after local resection to a high-grade subtype that recurs and progresses to metastatic, lethal disease. Although 80% of patients present at a non–muscle-invasive stage (Ta, T1, TIS) that may be managed endoscopically, recurrence rate reaches 61% at 1 year and 78% at 5 years. As a result of its high recurrence rate and associated need for lifelong surveillance and repeat resections, the health care costs for bladder cancer are among the highest of all malignancies.
White light cystoscopy (WLC) is the standard for evaluation of bladder urothelium. In the office setting, flexible cystoscopes are used for initial identification of suspected lesions and subsequent surveillance for recurrence. In the operating room, complete transurethral resection (TUR) with larger rigid cystoscopes is performed for tissue diagnosis and local staging. Despite its central role, WLC has well-recognized limitations. Although sufficient for the identification of papillary lesions, visual appearance under white light is unreliable for the determination of low- and high-grade cancer and cannot assess level of invasion. Additionally, nonpapillary and flat malignant lesions such as carcinoma in situ (CIS) can be difficult to differentiate from inflammation, with detection rates of CIS only 58% to 68% by WLC. Smaller or satellite tumors can be missed, which contributes to the up to 40% rate of residual bladder cancer found at the time of ‘second-look’ TUR. Finally, indistinct borders and difficult visualization of submucosal tumor margins during TUR can lead to incomplete tumor resection and understaging of bladder cancer. These limitations of WLC contribute to the increased risk of cancer persistence, recurrence, and in the case of high-grade bladder cancer, progression to metastatic lethal disease.
To address the shortcomings of WLC, several adjunctive optical imaging technologies have emerged with the goal to improve bladder cancer detection and resection ( Table 1 ). The imaging technologies can be broadly categorized based on their field of view. Photodynamic diagnosis (PDD) and narrow band imaging (NBI) are examples of macroscopic imaging modalities that survey a large area of mucosa, similar to WLC, but provide additional contrast enhancement to distinguish suspicious lesions from noncancerous mucosa. Microscopic modalities including optical coherence tomography (OCT) and confocal laser endomicroscopy (CLE) provide high-resolution, subsurface tissue characterization similar to histology and thus offer the potential for ‘optical biopsy’ of bladder cancer. Molecular imaging, through coupling of optical imaging technologies with fluorescently labeled binding agents (eg, antibodies), may enable real-time cancer imaging with molecular specificity. These technological advances have the potential to improve optical diagnosis and endoscopic management of bladder cancer. The most recent literature on adjunctive optical imaging technologies is reviewed, with consideration for the evaluation of high-grade bladder cancer highlighted.
| Name | Mechanism | Contrast Agent | Resolution | Depth | Scope or Probe (Diameter) | Status |
|---|---|---|---|---|---|---|
| PDD | Fluorescence | HAL | mm – cm | Surface | Standard rigid cystoscope | Clinical |
| NBI | Absorption | None | mm – cm | Surface | Flexible cystoscope (5.5 mm) or standard rigid cystoscope | Clinical |
| CLE | Fluorescence | Fluorescein | 1–3.5 μm | 120 μm | Probe (0.85–2.6 mm) | Clinical/investigational (in vivo) |
| OCT | Scattering | None | 10–20 μm | ∼2.5 mm | Probe (2.7 mm) | Clinical/investigational (in vivo) |
| Raman | Scattering | Optional (SERS) | — | 2 mm | Probe (2.1 mm) | Investigational (in vivo) |
| UV | Fluorescence | None | mm – cm | Surface | Probe (3 mm) | Investigational (in vivo) |
| SFE | Reflectance + fluorescence | None | mm – cm | Surface | Scope/Probe (1.2 mm) | Investigational (ex vivo) |
Introduction
Bladder cancer is the sixth most common cancer in the United States with 74,690 new cases and 15,580 deaths expected in 2014. The natural history of bladder cancer is heterogeneous, ranging from low-grade variant that does not recur after local resection to a high-grade subtype that recurs and progresses to metastatic, lethal disease. Although 80% of patients present at a non–muscle-invasive stage (Ta, T1, TIS) that may be managed endoscopically, recurrence rate reaches 61% at 1 year and 78% at 5 years. As a result of its high recurrence rate and associated need for lifelong surveillance and repeat resections, the health care costs for bladder cancer are among the highest of all malignancies.
White light cystoscopy (WLC) is the standard for evaluation of bladder urothelium. In the office setting, flexible cystoscopes are used for initial identification of suspected lesions and subsequent surveillance for recurrence. In the operating room, complete transurethral resection (TUR) with larger rigid cystoscopes is performed for tissue diagnosis and local staging. Despite its central role, WLC has well-recognized limitations. Although sufficient for the identification of papillary lesions, visual appearance under white light is unreliable for the determination of low- and high-grade cancer and cannot assess level of invasion. Additionally, nonpapillary and flat malignant lesions such as carcinoma in situ (CIS) can be difficult to differentiate from inflammation, with detection rates of CIS only 58% to 68% by WLC. Smaller or satellite tumors can be missed, which contributes to the up to 40% rate of residual bladder cancer found at the time of ‘second-look’ TUR. Finally, indistinct borders and difficult visualization of submucosal tumor margins during TUR can lead to incomplete tumor resection and understaging of bladder cancer. These limitations of WLC contribute to the increased risk of cancer persistence, recurrence, and in the case of high-grade bladder cancer, progression to metastatic lethal disease.
To address the shortcomings of WLC, several adjunctive optical imaging technologies have emerged with the goal to improve bladder cancer detection and resection ( Table 1 ). The imaging technologies can be broadly categorized based on their field of view. Photodynamic diagnosis (PDD) and narrow band imaging (NBI) are examples of macroscopic imaging modalities that survey a large area of mucosa, similar to WLC, but provide additional contrast enhancement to distinguish suspicious lesions from noncancerous mucosa. Microscopic modalities including optical coherence tomography (OCT) and confocal laser endomicroscopy (CLE) provide high-resolution, subsurface tissue characterization similar to histology and thus offer the potential for ‘optical biopsy’ of bladder cancer. Molecular imaging, through coupling of optical imaging technologies with fluorescently labeled binding agents (eg, antibodies), may enable real-time cancer imaging with molecular specificity. These technological advances have the potential to improve optical diagnosis and endoscopic management of bladder cancer. The most recent literature on adjunctive optical imaging technologies is reviewed, with consideration for the evaluation of high-grade bladder cancer highlighted.
| Name | Mechanism | Contrast Agent | Resolution | Depth | Scope or Probe (Diameter) | Status |
|---|---|---|---|---|---|---|
| PDD | Fluorescence | HAL | mm – cm | Surface | Standard rigid cystoscope | Clinical |
| NBI | Absorption | None | mm – cm | Surface | Flexible cystoscope (5.5 mm) or standard rigid cystoscope | Clinical |
| CLE | Fluorescence | Fluorescein | 1–3.5 μm | 120 μm | Probe (0.85–2.6 mm) | Clinical/investigational (in vivo) |
| OCT | Scattering | None | 10–20 μm | ∼2.5 mm | Probe (2.7 mm) | Clinical/investigational (in vivo) |
| Raman | Scattering | Optional (SERS) | — | 2 mm | Probe (2.1 mm) | Investigational (in vivo) |
| UV | Fluorescence | None | mm – cm | Surface | Probe (3 mm) | Investigational (in vivo) |
| SFE | Reflectance + fluorescence | None | mm – cm | Surface | Scope/Probe (1.2 mm) | Investigational (ex vivo) |
Photodynamic diagnosis
PDD, also known as fluorescence cystoscopy or blue light cystoscopy, provides wide-field fluorescence imaging of the bladder with a field of view comparable to WLC. PDD requires preoperative intravesical administration of a photosensitive protoporphyrin IX precursor as the contrast agent, a blue light source that illuminates at 375 to 440 nm, and a specialized lens and camera head. Once taken up by the bladder urothelium, the protoporphyrin accumulates preferentially in neoplastic cells and emits a fluorescence in the red part of the spectrum under blue light excitation, allowing visualization of the tumor ( Fig. 1 ). Two protoporphyrin analogues, 5-aminolevulinic acid and its ester derivative hexaminolevulinate, have been investigated clinically. Hexaminolevulinate is the more potent analog, with greater local bioavailability and superior fluorescence intensity, and is approved for single clinical use in Europe and the United States for patients with suspected or known bladder cancer. Owing to a false-positive fluorescence from inflammatory lesions, previous biopsy sites, or in patients previously treated with bacillus Calmette-Guérin, hexaminolevulinate is not approved currently for patients who received intravesical immunotherapy or chemotherapy within 90 days.
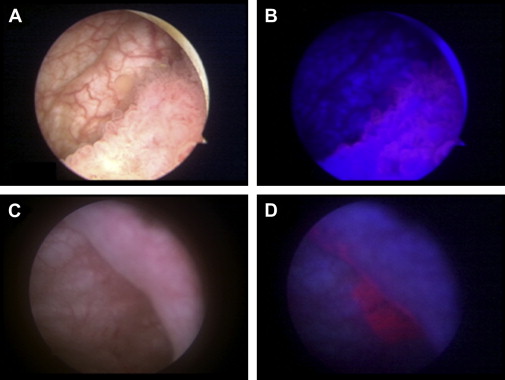
Multi-institutional, randomized, clinical studies have demonstrated that PDD improves the detection of papillary bladder tumors. Although PDD does not distinguish high-grade from low-grade bladder cancer, several studies have shown that PDD has an increased rate of detection of flat-appearing CIS. In a meta-analysis of 3 phase III studies, the detection of CIS was significantly higher by PDD plus WLC compared with WLC alone (87% vs 75% pooled sensitivity; P = .006). Additionally, several meta-analyses have found significantly reduced residual tumor rates in patients treated with PDD, with relative risk of residual tumor 2.77-fold higher for WLC compared with PDD.
The recurrence rate of PDD-guided TUR of bladder tumor remains to be determined. In a meta-analysis that reviewed raw data from prospective studies on 1345 patients with known or suspected non–muscle-invasive bladder cancer, overall recurrence rates up to 12 months were significantly lower with PDD compared with WLC (34.5% vs 45.4% pooled sensitivity; P = .006) and independent of the level of risk. However, a prospective randomized multi-institutional trial found no difference in tumor recurrence and progression between PDD and WLC in patients with non–muscle-invasive bladder cancer. Additional studies with longer follow-up time periods are needed to better define the optimal indications for PDD use and evaluate the long-term efficacy of PDD with regard to recurrence-free and progression-free survival.
Narrow band imaging
NBI is a high-resolution wide-field endoscopic technique that improves detection of bladder neoplasia through enhanced visualization of mucosal and submucosal vasculature without the use of exogenous dye. NBI devices (Olympus Corp, Tokyo, Japan) filter out the red spectrum from white light, with the resultant blue (415 nm) and green (540 nm) spectra absorbed by hemoglobin, thus highlighting the contrast between capillaries and mucosa. Under NBI, the more vascularized CIS or tumor areas are accentuated in appearance as green or brown ( Fig. 2 ). NBI is approved for clinical use in the United States and is available either in an integrated videocystoscope or through a camera head that can be attached to standard cystoscopes. These devices include a toggling functionality between WLC and NBI, thereby facilitating rapid and real-time evaluation of suspected lesions. Although NBI provides a subjective impression of abnormal areas of bladder mucosa, there does not seem to be a significant difference in detection rate of bladder tumor between new and experienced users.
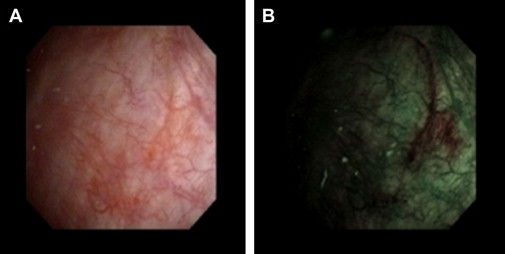
In a recent meta-analysis of 8 studies including 1022 patients, the detection of bladder cancer was higher by NBI compared with WLC on a per-person basis (94% vs 85% pooled sensitivity) and a per-lesion basis (95% vs 75% pooled sensitivity); however, the pooled specificity on a per-lesion basis was lower by NBI compared with WLC (55% vs 72%).
Similar to PDD, NBI does not distinguish bladder cancer grade, but does improve CIS detection. In a study of 427 patients that compared NBI plus WLC with WLC alone for recurrent bladder cancer, the detection of CIS was significantly improved by NBI over WLC (100% vs 83% sensitivity). A single-center, prospective, randomized, controlled trial involving 220 patients with bulky non–muscle-invasive bladder tumor demonstrated an improved detection rate of CIS for NBI compared with WLC (95% vs 68%). A multicenter, prospective study reported a significantly increased sensitivity for the detection of CIS from 50% for WLC to 90% for NBI in 104 patients.
In a recent, single-center, randomized, controlled trial to assess whether NBI improved TUR of bladder tumors in 254 patients with 2-year follow-up, a reduced recurrence rate (22% vs 33%; P = .05) and improved recurrence-free survival (22 vs 19 months; P = .02) were reported by NBI compared with WLC. A multicenter, randomized, controlled trial to compare the recurrence rate at 1 year between NBI- and WLC-assisted TUR of bladder tumor in patients with non–muscle-invasive bladder cancer is ongoing.
Confocal laser endomicroscopy
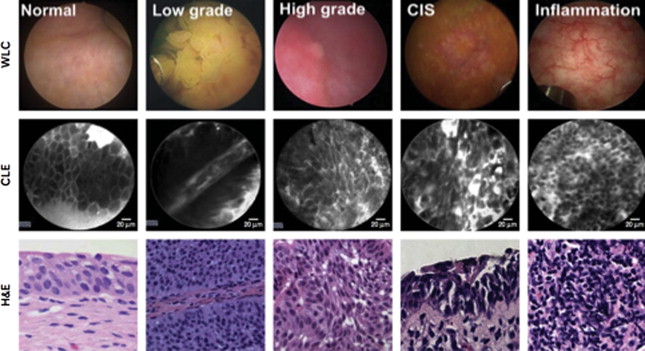
CLE is an optical biopsy technology that provides dynamic, high-resolution microscopy of mucosal lesions. Recently approved for clinical use in the urinary tract, image acquisition is performed through fiber optic probes ranging from 0.85 to 2.6 mm in diameter, compatible with working channels of standard cystoscopes. Fluorescein, a US Food and Drug Administration–approved drug, is used as the contrast agent and can be administered intravesically or intravenously with minimal toxicity. In the current CLE clinical system (Mauna Kea Technologies, Paris, France), illuminating light from a 488-nm laser fiber source is focused by an objective lens, with scattered light from the in-focus tissue plane converged back into the fiber and subsequent signal processing into an image. CLE provides the highest spatial resolution (1–5 μm) of clinically available technologies, with images comparable with conventional histopathology. Video sequences are acquiring at 12 frames per second, allowing for real-time dynamic imaging of physiologic processes such as vascular flow.
Given spatial resolution sufficient to resolve microarchitectural and cellular features, CLE is capable of differentiating high-grade and low-grade bladder cancer. After the initial pilot study that demonstrated the feasibility of using CLE in ex vivo bladders after cystectomy, CLE was conducted in 27 patients undergoing cystoscopy and TUR and differences between normal urothelium, low-grade tumors and high-grade tumors were visualized. In normal urothelium, larger umbrella cells were seen most superficially followed by organized smaller intermediate cells with distinct cell borders. Low-grade papillary tumors demonstrated densely arranged but normal-shaped small cells extending outward from fibrovascular cores, whereas high-grade tumors and CIS showed markedly irregular architecture and cellular pleomorphism ( Fig. 3 ).