Barrett’s esophagus (BE) is present in up to 5.6% of the US population and is the precursor lesion for esophageal adenocarcinoma. Surveillance endoscopy is the primary management approach for BE. However, standard protocol biopsies have been associated with significant miss rates of dysplastic lesions in patients with BE. Thus, a variety of methods to optimize the imaging of BE have been developed to improve the efficiency and diagnostic yield of surveillance endoscopy in detecting early neoplasia. These techniques use changes that occur at macroscopic, microscopic, and subcellular levels in early neoplasia and are the focus of this article.
Key points
- •
A careful endoscopic examination using high-resolution white light endoscopy (HR-WLE) is the current standard in the evaluation of Barrett’s esophagus (BE).
- •
Advances in imaging technology have led to promising new endoscopic tools that may improve the detection of BE and early neoplasia.
- •
The optimal combination of wide-field surveillance and focal high-resolution imaging for BE has yet to be determined.
Introduction
BE is estimated to be present in up to 5.6% of the US population and is the precursor lesion for esophageal adenocarcinoma, which has a poor 5-year survival rate of 17%. Surveillance endoscopy is now the primary management approach for BE, with 4-quadrant biopsies being obtained every 1 to 2 cm at designated intervals in an attempt to identify dysplasia and early neoplasia. The goal of this approach is to treat patients with identified dysplastic or neoplastic lesions with endoscopic eradication therapies in lieu of surgery or chemoradiation, which is used for patients presenting with more advanced cancers. However, standard protocol biopsies have been associated with a miss rate of up to 57% for dysplastic/neoplastic lesions in patients with BE. Thus, a variety of methods to optimize the imaging of BE have been developed to improve the efficiency and diagnostic yield of surveillance endoscopy in detecting early neoplasia ( Table 1 ). These techniques use changes that occur at macroscopic, microscopic, and subcellular levels in early neoplasia and are the focus of this article.
| Technology | Advantages | Disadvantages |
|---|---|---|
| Standard WLE | Wide-field imaging, widely available, no contrast | Limited sensitivity and specificity |
| High resolution WLE | Wide-field imaging, improved image quality, no contrast | Cost of upgrading entire endoscopy system |
| Dye-based chromoendoscopy | Wide-field imaging, mucosal enhancement | Time consuming, tedious, requires contrast, contrast may be harmful |
| Optical chromoendoscopy | Wide-field imaging, mucosal enhancement, no contrast | Unclear if there is added benefit to high-resolution WLE alone |
| AFI | Wide-field imaging, high sensitivity | High false-positive rate |
| CLE | In vivo histology, probe can be used in any endoscope | Near-field imaging, expensive, requires fluorescein, interpretation of imaging |
| OCT | In vivo assessment of tissue architecture, no contrast; ability for subsurface imaging | Near-field imaging, early in development |
| HRME | In vivo histology, can be used in any endoscope | Near-field imaging, requires contrast, not commercially available |
| Endocytoscopy | In vivo histology, probe can be used in any therapeutic endoscope | Near-field imaging, expensive, requires contrast agents, labor-intensive, not commercially available |
Introduction
BE is estimated to be present in up to 5.6% of the US population and is the precursor lesion for esophageal adenocarcinoma, which has a poor 5-year survival rate of 17%. Surveillance endoscopy is now the primary management approach for BE, with 4-quadrant biopsies being obtained every 1 to 2 cm at designated intervals in an attempt to identify dysplasia and early neoplasia. The goal of this approach is to treat patients with identified dysplastic or neoplastic lesions with endoscopic eradication therapies in lieu of surgery or chemoradiation, which is used for patients presenting with more advanced cancers. However, standard protocol biopsies have been associated with a miss rate of up to 57% for dysplastic/neoplastic lesions in patients with BE. Thus, a variety of methods to optimize the imaging of BE have been developed to improve the efficiency and diagnostic yield of surveillance endoscopy in detecting early neoplasia ( Table 1 ). These techniques use changes that occur at macroscopic, microscopic, and subcellular levels in early neoplasia and are the focus of this article.
| Technology | Advantages | Disadvantages |
|---|---|---|
| Standard WLE | Wide-field imaging, widely available, no contrast | Limited sensitivity and specificity |
| High resolution WLE | Wide-field imaging, improved image quality, no contrast | Cost of upgrading entire endoscopy system |
| Dye-based chromoendoscopy | Wide-field imaging, mucosal enhancement | Time consuming, tedious, requires contrast, contrast may be harmful |
| Optical chromoendoscopy | Wide-field imaging, mucosal enhancement, no contrast | Unclear if there is added benefit to high-resolution WLE alone |
| AFI | Wide-field imaging, high sensitivity | High false-positive rate |
| CLE | In vivo histology, probe can be used in any endoscope | Near-field imaging, expensive, requires fluorescein, interpretation of imaging |
| OCT | In vivo assessment of tissue architecture, no contrast; ability for subsurface imaging | Near-field imaging, early in development |
| HRME | In vivo histology, can be used in any endoscope | Near-field imaging, requires contrast, not commercially available |
| Endocytoscopy | In vivo histology, probe can be used in any therapeutic endoscope | Near-field imaging, expensive, requires contrast agents, labor-intensive, not commercially available |
High-definition white light examination
BE is a diagnosis made based on endoscopic visualization and histologic confirmation. Careful inspection of the esophageal mucosa on upper endoscopy is essential in the detection and surveillance of BE. Consequently, the quality of endoscopic imaging has diagnostic implications on the gastroenterologist’s ability to detect and identify intestinal metaplasia, dysplasia, and even early neoplasia.
The quality of endoscopic visualization depends on image resolution and magnification. Resolution refers to the amount of detail within an image and is a function of the pixel density. As the number of pixels increases, the resolution correspondingly improves, providing sharper, more defined, and more detailed images. Standard definition (SD) endoscopes are equipped with charge-coupled device (CCD) chips, which can produce images with up to 400,000 pixels that are then displayed on a traditional display in a 4:3 (width:height) aspect ratio. Technologic advances now enable smaller CCD chips that are capable of producing images with much higher resolution. High-definition or high-resolution endoscopes can capture images with more than 800,000 to 2.1 million pixels that can be displayed on monitors with a 16:9 aspect ratio ( Fig. 1 ). The superior imaging quality of the high-resolution endoscopes is 2- to 5-fold better than SD white light endoscopy (WLE), enabling improved visualization of the mucosal surface in BE.
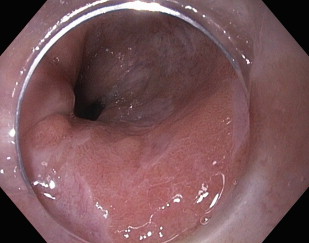
The other essential component of endoscopic visualization is image magnification. Standard endoscopes are designed to magnify the video image by 30 to 35 times. Many endoscopes may also have a built-in 1.5× to 2× digital zoom, an electronic zoom that brings the center of the image closer without improvement in resolution. This feature should not be confused with specially designed high-definition magnification endoscopes that can optically magnify images by 70 to 140 times their normal size, 2 to 4 times the capability of the standard endoscope. High-resolution magnification endoscopes provide detailed imaging of the mucosal patterns while maintaining image resolution. These highly specialized endoscopes have been studied, primarily in combination with chromoendoscopy, with regards to their ability to enhance the visualization of the mucosal pit patterns and microvasculature morphology of BE.
Expert opinion recommends that HR-WLE should be the minimum standard in the evaluation of patients with BE. However, in their consensus statement, the investigators acknowledge that there is limited high-quality evidence to support this recommendation. At present, there are no randomized studies that directly compare high-resolution to standard WLE in the management of BE. However, the notion that higher resolution may be more sensitive for detecting early neoplastic changes in BE may be inferred from several studies in which standard WLE has been compared with HR-WLE with chromoendoscopy. In a prospective, blinded, tandem endoscopy study, patients with BE with known dysplasia underwent SD endoscopy followed by HR-WLE with narrow band imaging (NBI; Olympus, Center Valley, PA, USA) by 2 different endoscopists. HR-WLE with NBI was superior to SD endoscopy in identifying patients with dysplasia as well as detecting higher grades of dysplasia. The study design makes it difficult to determine if the improved detection of early neoplastic changes can be attributed to the HR-WLE, mucosal enhancement with NBI, or the combination of the 2 modalities. Subsequent studies suggest that most of the benefit is derived from the HR-WLE alone and that the added value of using either dye-based or optical chromoendoscopy is uncertain. In a prospective, randomized, crossover study comparing HR-WLE with either indigo carmine chromoendoscopy or NBI, HR-WLE was able to successfully identify subtle dysplastic and early neoplastic changes in BE in 11 of 14 patients (79%). Targeted biopsies using indigo carmine and NBI had limited added benefit to high-resolution endoscopy alone (79% for both procedures, P = 1). Another study comparing HR-WLE still images of dysplastic BE with and without indigo carmine, acetic acid, and NBI found that these additional imaging techniques did not improve the identification of early neoplasia compared with the HR-WLE alone (diagnostic yields: HR-WLE alone, 86%; indigo carmine, 70%; acetic acid, 83%; and NBI, 84%). Although direct head-to-head comparison studies are limited, HR-WLE seems to be more sensitive than standard WLE in the detection of BE and its associated early neoplasia.
The role of high-resolution magnification endoscopy alone in BE is unclear. The high-power magnification capability of these scopes is an adjunct usually used in concert with chromoendoscopy (dye based and optical) to closely visualize the mucosal and microvascular patterns of BE. The first use of magnification endoscopy in BE was described in 1994 in which Lugol solution and indigo carmine were used to identify a villiform surface mucosal pattern, which correlated with histologic intestinal metaplasia. Subsequent studies have similarly used indigo carmine or methylene blue with high-magnification endoscopy for identification of BE and surveillance of dysplasia and neoplasia. NBI has also been combined with high-resolution magnification endoscopy in the management of BE. A meta-analysis has shown that NBI with magnification can accurately diagnose high-grade dysplasia (HGD) in BE. One small study required 5 expert endoscopists to examine images of BE taken with high-resolution magnification white light and NBI to predict final histopathology. NBI with magnification was superior to high-resolution magnification alone in identifying both nondysplastic BE and HGD in this cohort. Further studies are necessary before any specific conclusions can be made about the role of high-resolution magnification endoscopy in BE.
In summary, despite the limited evidence, it seems reasonable that HR-WLE should be the minimum standard in the initial evaluation and subsequent surveillance of BE. The additional benefit of adding magnification to HR-WLE is uncertain, as this modality has only been studied in conjunction with chromoendoscopy.
Dye-based chromoendoscopy
Dye-based chromoendoscopy is a wide-field diagnostic imaging tool in which a dye or chemical solution is sprayed onto the gastrointestinal mucosa to enhance visualization of the subtle mucosal and microvascular patterns. In many studies, dye-based chromoendoscopy is often used in combination with high-resolution magnification endoscopes to image the mucosa in exquisite detail. Various stains have proved to be useful in the evaluation of BE by highlighting features of intestinal metaplasia, dysplasia, and early neoplasia that may not be readily apparent with WLE ( Fig. 2 ). The different types of dyes used in BE as chromoendoscopy agents include methylene blue, indigo carmine, and acetic acid, each with its own unique properties to facilitate detection of mucosal abnormalities ( Table 2 ).
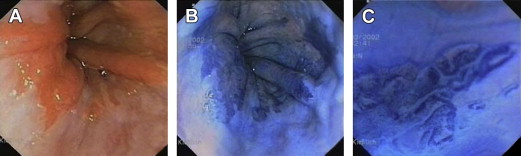
| Chromoendoscopy Agent | Type of Dye | What Does It Stain? | What Does It Not Stain? | Barrett’s Esophagus | Early Neoplasia |
|---|---|---|---|---|---|
| Methylene blue | Vital stain | Intestinal mucosa | Squamous epithelium | Regular, homogenous, dark blue colored mucosa | Irregular, heterogenous, varying dark and light blue colored mucosa |
| Indigo carmine | Contrast dye | Not absorbed; pools in mucosal grooves and crevices | Does not stain mucosa | Villiform pattern with tortuous, thick villi | Distortion and irregularity of cerebriform and villous pattern |
| Acetic acid | Colorless stain | Enhances mucosal surface and vascular patterns | All mucosa stained | Fine villiform appearance with regular shape and arrangement | Irregular surface pattern, increased vascularity |
Spraying catheters are typically used to apply the dye in a fine mist covering the esophageal mucosa. The staining dye solution should be sprayed evenly to provide a consistent light coating on the mucosal surface. Excessive spraying can lead to pooling of the dye solution in dependent areas, making the underlying mucosa difficult to interpret. In such instances, any excess dye should be washed away to provide better visualization.
Methylene blue is a vital stain that is preferentially absorbed by intestinal and colonic epithelium. Hence, the intestinal metaplasia of BE stands out from the background of normal squamous epithelium. Methylene blue chromoendoscopy is generally preceded by applying 10% N -acetylcysteine to clean the surface mucosa, followed by spraying 0.5% methylene blue onto the mucosa, waiting 2 to 3 minutes to allow absorption, and subsequently irrigating with water. The methylene blue staining begins to highlight absorptive mucosa within 2 to 3 minutes and wears off after 20 minutes. In BE, the intestinal metaplasia preferentially absorbs the dye, transitioning from the typical salmon-colored mucosa to mucosa with a dark blue hue with a regular, homogeneous pattern. Irregular, heterogeneous staining of the mucosa with varying dark and light blue discoloration can be a cause of concern for dysplastic BE. Initial studies found methylene blue to be an effective tool in targeting intestinal metaplasia and dysplasia, but subsequent studies have had equivocal results. A meta-analysis ultimately concluded that chromoendoscopy using methylene blue provided no benefit over standard 4-quadrant biopsies in detecting intestinal metaplasia and dysplasia in patients with BE. In addition, in a cohort of patients undergoing surveillance for BE, methylene blue was found to induce oxidative damage to DNA when photosensitized with white light. Although the clinical significance of this finding is unclear, the potential to accelerate carcinogenesis in a well-recognized premalignant condition has understandably raised concerns regarding the safety profile of methylene blue in BE. Given the lack of clinical efficacy, the time-consuming spray protocol, and the potential risks of methylene blue chromoendoscopy, this technique is no longer popular.
Chromoendoscopy using indigo carmine may be useful in the evaluation of BE and its associated high-risk lesions. Indigo carmine was used in the first successful demonstration of dye-based chromoendoscopy in the evaluation of BE. Indigo carmine is unique in that the dye is not absorbed by the epithelium. Rather, the solution settles between the pits and grooves of the surface epithelium, providing a detailed topographic image of the mucosa. Using high-resolution magnification endoscopy with indigo carmine, targeted biopsies of ridged and villous-appearing mucosa accurately identified intestinal metaplasia in 57 of 62 patients (97%), whereas irregular and distorted mucosa was associated with HGD in 6 of 6 patients (100%). In a subsequent prospective multicenter study of 56 patients with BE, targeted biopsies using high-resolution magnification endoscopy in conjunction with indigo carmine proved to have high sensitivity (83%) and specificity (88%) for HGD while taking less time than performing standard random biopsies.
Acetic acid is a colorless dye and reacts with the epithelium to enhance the surface mucosal pattern. A 1.5% to 3% acetic acid solution is sprayed onto the mucosa, causing whitish discoloration of the epithelium, and examined under high-resolution magnification endoscopy. Close inspection of the surface pit pattern in areas of suspected BE can distinguish normal esophagus from intestinal metaplasia and neoplasia, with a high correlation with final histology (r = 0.98). Although no randomized studies have been performed to date, acetic acid has been shown to significantly improve the detection of neoplasia as compared with WLE with routine random biopsies.
For a variety of reasons, dye-based chromoendoscopy has not gained widespread clinical use, including a perception that the technique is time consuming and tedious, the concurrent need for high-magnification endoscopy, and concerns regarding the potential to cause bodily harm to the patient. In addition, no standardized classification criteria have been established for dye-based chromoendoscopy, leading to wide variability in sensitivity and specificity.
In summary, dye-based chromoendoscopy seems to have a limited role in the evaluation of patients with suspected or established BE. This technique has been largely supplanted by optical chromoendoscopy because of the ease and safety of this method.
Optical chromoendoscopy
Optical chromoendoscopy is a wide-field diagnostic tool that uses light filters and computer processing technology within the endoscope to enhance visualization of the esophageal mucosa. The principle is similar to that of traditional dye-based chromoendoscopy but without the unwieldy process of spraying and instilling dyes. There are 3 commercially available forms of optical chromoendoscopy as designed by each of the major endoscope manufacturers incorporated into the endoscopes. NBI filters white light into 2 specific wavelengths, 415 nm and 540 nm, that are strongly absorbed by hemoglobin. The filtered light serves to highlight both superficial veins and capillaries along the surface, as well as deeper blood vessels within the mucosa. Fujinon intelligent color enhancement (FICE; Fujinon, Inc, Wayne, NJ, USA) and I-Scan (Pentax Medical, Montvale, NJ, USA) use proprietary postimage acquisition processing technology to modify the white light image and create an enhanced view of the esophageal mucosa. Among these 3 modalities, NBI is most commonly used and has been studied more rigorously than FICE or I-Scan. The few studies that have evaluated FICE and I-SCAN were used in conjunction with acetic acid chromoendoscopy. Therefore, NBI is the focus of the following discussion.
BE and HGD have characteristic findings on optical chromoendoscopy that are readily recognizable by the abnormal vascular and mucosal patterns. Intestinal metaplasia has a flat, villous-appearing mucosal pattern with long branching vessels, whereas HGD is described as having disrupted mucosa with irregular blood vessels ( Fig. 3 ). NBI has consistently been shown to detect intestinal metaplasia and HGD with a high degree of accuracy when correlated with histology. No formal classification of mucosal and vascular patterns have been standardized or validated, which has likely limited the routine clinical use of optical chromoendoscopy in evaluating patients with BE.
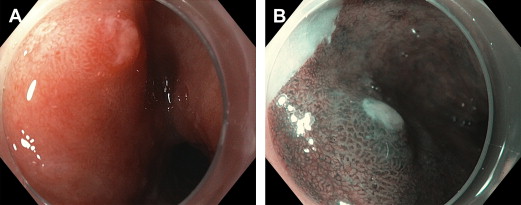
Studies comparing NBI to WLE in detecting dysplasia have had equivocal results ( Table 3 ). In a prospective tandem study, NBI was superior to SD WLE as NBI was able to detect more patients with dysplasia and higher grades of dysplasia. However, NBI was unable to improve the diagnostic yield of HGD or early neoplasia when compared with HR-WLE alone. In the most comprehensive study comparing NBI with HR-WLE, an international, randomized controlled crossover trial found that NBI with targeted biopsies had the same detection rate of intestinal metaplasia as HR-WLE with standard protocol biopsies, detecting a higher proportion of dysplasia (30% vs 21%, P = .01) using fewer biopsies per patient (3.6 vs 7.6, P <.0001). A meta-analysis of 8 studies found favorable test characteristics of NBI in the diagnosis of HGD (sensitivity 96%, specificity 94%) and BE (sensitivity 95%, specificity 65%). Evidence suggests that NBI may increase the diagnostic yield of targeted biopsies of early neoplasia in BE and may be useful as an adjunctive tool to HR-WLE.
| Study | Study Design | Patients, n | Lesions, n | Histologic Reference Standard | Results |
|---|---|---|---|---|---|
| Sharma et al, 2013 | Crossover trial | 123 | 977 | BE, dysplasia | BE: NBI equivalent to WLE Dysplasia: NBI detected more dysplasia than WLE ( P = .01) |
| Wolfsen et al, 2008 | RCT (tandem) | 65 | — | BE, dysplasia | NBI detected more dysplasia than WLE (57% vs 43% patients) |
| Singh et al, 2009 | Cross-sectional | 109 | 1021 | BE, HGD | NBI grading correlated with histology in 903 of 1021 lesions (87.9%) |
| Curvers et al, 2008 | Cross-sectional | 84 | 165 | HGD | NBI used to decrease the false-positive rate of autofluorescence in detecting HGD from 81% to 26% |
| Sharma et al, 2006 | Cross-sectional | 51 | 204 | BE, HGD | BE: NBI detects with sensitivity 93.5%, specificity 86.7%, PPV 94.7% HGD: NBI detects with sensitivity 100%, specificity 98.7%, PPV 95.3% |
| Goda et al, 2007 | Cross-sectional | 58 | 217 | BE, carcinoma | 6 adenocarcinoma sites had irregular mucosal and capillary patterns on NBI |
| Anagnostopoulos et al, 2007 | Cross-sectional | 50 | 344 | BE, HGD | BE: NBI detects with sensitivity 100%, specificity 78.8%, PPV 93.5%, NPV 100% HGD: NBI detects with sensitivity 90%, specificity 100%, PPV 99.2%, NPV 100% |
| Kara et al, 2006 | Cross-sectional | 63 | 161 | BE, HGD | HGD: NBI detects with sensitivity 94%, specificity 76%, PPV 64%, NPV 98% |
| Kara et al, 2006 | Cross-sectional | 20 | 47 | HGD | NBI used to decrease the false-positive rate of autofluorescence in detecting HGD from 40% to 10% |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





