CHAPTER 15 The influence of the composition of the hormonal milieu and the timing of exposure on the establishment of gender identity are not fully understood. Most agree that there is a complex interaction between the genetic blueprint and hormonal regulation of genetic expression that influences psychological elements, resulting in self-perception of gender. Hormones, and specifically sex hormones (androgens and estrogens), can have a profound effect on the perception of one’s gender. For example, intrauterine androgen exposure of XX females may predispose to gender identity disorders.1 In addition, XY males exposed to male-typical prenatal androgen have a high likelihood of declaring a male sexual identity, even when raised as female. However, inappropriate prenatal androgen exposure demonstrated unpredictable sexual identification.2 The prevalence of disorders of sex development is estimated at 0.1% to 2% of the global population, and of those, 8.5% to 20% present with gender dysphoria.3 Gender dysphoria is a psychiatric diagnosis, which should only be made after a careful endocrine evaluation and other disorders of sexual differentiation have been excluded. Many syndromes affecting sexual development present at an early age, which prompt evaluation by the pediatrician. The clinical manifestations include clitoromegaly, penile agenesis, bilateral or unilateral cryptorchidism, posterior labial fusion, and hypospadias. Obtaining a thorough family history of maternal virilization during gestation, prenatal exposure to androgens, infertility, miscarriages, or consanguinity is important in understanding the etiologic factors for definable causes of gender dysphoria. The initial assessment of any patient should include a thorough clinical history, physical examination, determination of sex chromosomes, pelvic/abdominal ultrasonography, measurement of 17-hydroxyprogesterone, testosterone, gonadotropins, anti-Müllerian hormone, serum electrolytes, and urinalysis.4 According to the International Consensus Conference, disorders of sex development can be categorized as 46,XX or 46,XY or a mixed sex chromosome pattern5,6 (Table 15-1). As stated in the “World Professional Association for Transgender Health Standards of Care (WPATH SOC) for the Health of Transsexual, Transgender, and Gender Nonconforming People,” transgender/transsexual patients need to fulfill eligibility and readiness criteria before proceeding with cross-sex hormone therapy.7 Patients undergoing sex reassignment therapy must understand the reversible and irreversible effects of cross-sex hormones. A comprehensive discussion of the patient’s expectations should be done by the treating endocrinologist before the patient starts treatment.7 The criteria suggested for transgender hormone therapy for transgender adults are as follows: 1. The treating physician should confirm that the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5), or the tenth revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) criteria for gender identity disorder or transexualism are present. 2. The patient should have an understanding of what hormonal sex reassignment therapy can and cannot accomplish and the social benefits and risks. 4. Documented real-life experience should be undertaken for at least 3 months before the administration of hormones or a period of psychotherapy of a duration specified by the mental health professional after the initial evaluation (usually a minimum of 3 months). After the eligibility criteria have been fulfilled, the readiness of the transgender patient for hormonal therapy should be evaluated. The WPATH SOC document three key elements: 1. The patient has had further consolidation of gender identity during the real-life experience or psychotherapy. 2. The patient has been evaluated for other mental health conditions (for example, sociopathy, substance abuse, psychosis, and suicidality). 3. Hormones are likely to be taken in a responsible manner. As outlined by the 2009 Endocrine Society guidelines on endocrine treatment of transgender individuals, adverse mental health events can be prevented, if the transgender patient undergoing hormonal sex reassignment has a clear understanding of the mental and physical changes that will follow after hormonal therapy is initiated.8 It is also essential for the caregiver to provide counseling with regard to the effects of cross-sex therapy on fertility and the available options to preserve fertility for the future. It has been our experience that patients, because of economic reasons or inaccessibility of proper endocrinologic consultation, frequently seek to obtain the hormones without physician supervision through various sources (for example, Internet purchasing). Although this is illegal in most locales, some patients find that this is the only way to obtain hormones. Unfortunately, the quality of the hormone preparations is questionable, and there is no monitoring of the patient for adverse events. We think that it is better for the patient to obtain the medications through a physician rather than without physician supervision, even if it means that not all the previous criteria are met. Transgender patients seek hormone therapy to achieve anatomic and psychological changes that will make them feel and appear more like members of their aspired-to-be gender. Using the same principle for hormone replacement therapy in the hypogonadal patient, the main objectives of hormonal therapy are to induce the development of secondary sex characteristics of the reassigned gender and to suppress the individual’s genetic sex characteristics by reducing and replacing endogenous hormones. Hormone treatment can be acceptably safe and provide improvement in the quality of life and mental well-being.9 Areas that should be covered before the initiation of cross-sex hormone therapy by the treating endocrinologist are the risks and benefits of hormone therapy, the presence of comorbidities that can be exacerbated by hormonal treatment, and the relative contraindications to hormonal therapy (liver disease, diabetes, and metabolic syndrome). Smoking cessation is highly recommended to avoid an increased risk of cardiovascular disease and thromboembolism. Table 15-2 Hormone Therapy Options for Female-to-Male Patients
Adult Hormone Therapy in Transgender Patients
Key Points
 The treatment and care of transgender individuals are now common practice in medicine, which requires medical personnel trained and experienced in the health needs of the transgender population.
The treatment and care of transgender individuals are now common practice in medicine, which requires medical personnel trained and experienced in the health needs of the transgender population.
 Hormone treatment should be administered to maximize the safest and most effective transition of desired sexual characteristics.
Hormone treatment should be administered to maximize the safest and most effective transition of desired sexual characteristics.
 Data on the effectiveness of cross-hormonal therapy protocols in the adult transgender population are limited and based on a few, nonrandomized studies.
Data on the effectiveness of cross-hormonal therapy protocols in the adult transgender population are limited and based on a few, nonrandomized studies.
 The significant consequences of male-to-female and female-to-male treatment affect cardiovascular disease, hormone-related cancers, and bone health.
The significant consequences of male-to-female and female-to-male treatment affect cardiovascular disease, hormone-related cancers, and bone health.
 Perioperative management of patients may require brief cessation of hormone therapy.
Perioperative management of patients may require brief cessation of hormone therapy.
 Reproductive function should be discussed with the patient and planned for appropriately.
Reproductive function should be discussed with the patient and planned for appropriately.
Hormonal Sex Reassignment
Initiation
Hormone Therapy
Hormone Therapy | Dose | Comments |
Testosterone enanthate or cypionate | 100-200 mg IM every 2 wk or 50-100 mg IM weekly | Monitor levels midway between injections |
Testosterone undecanoate | 1000 mg IM every 12 wk or 160-240 mg PO daily | Monitor levels midway between injections |
|
| Not available in the U.S. |
Testosterone gel | 2.5-10 mg daily | Higher incidence of breakthrough bleeding |
Testosterone patch | 2.5-7.5 mg daily | Patch can cause skin irritation |
Testosterone nasal (Natesto) | 5.5 mg per pump actuation; 1 pump actuation in each nostril TID | Monitor before next dose |
Female-to-Male Transgender Patients
The goal for female-to-male (FTM) transgender individuals is to induce virilization. To achieve this objective, there are different formulations and routes of administration of testosterone, including percutaneously administered gel, injectable intramuscular preparations, buccal tablets, and a nasal spray8,13 (Table 15-2). The main goal is to maintain a total serum testosterone level in the normal male range (350 to 1000 ng/dl).
There are several initiation protocols for androgen therapy, ranging from high doses of parenteral testosterone, with subsequent titration based on serum testosterone, or vice versa. Testosterone enanthate or cypionate can be delivered at doses of 100 to 200 mg every 2 weeks intramuscularly, or 1000 mg of testosterone undecanoate (not available in the United States) can be given every 12 weeks, with titration according to serum testosterone levels. After the desirable serum testosterone level is achieved, the patient can be switched to testosterone gel (25 to 50 mg/day).
Other protocols start with a lower dose of testosterone (for example, 100 mg of testosterone enanthate every 2 weeks) with subsequent adjustment. Therapy can also be initiated directly with testosterone gel, although one caveat is that the virilization achieved with transdermal methods is slower, given the lower serum levels achieved with this route. On the other hand, the risk of having a supraphysiologic serum concentration of testosterone is less common with this approach, thus decreasing the theoretical risk of adverse reactions.
The physical changes induced by androgen therapy include male pattern hair growth, increased muscle mass, an increase in fat mass, clitoromegaly, increased libido, deepening of the voice, and cessation of menses. However, permanent cessation of menses may require high doses of testosterone, which is rarely achieved with testosterone gel. If breakthrough uterine bleeding continues, concomitant therapy with a progestational agent may be needed, or endometrial ablation may be considered if hysterectomy is not desired. Recommended regimens include medroxyprogesterone acetate, 5 to 10 mg daily, or 17 alpha-ethinyl-3-desoxy-19-nortestosterone (Lynestrenol), 5 mg/day, which is not available in the United States. Other treatment options include depot medroxyprogesterone, 150 mg intramuscularly every 3 months, or a gonadotropin-releasing hormone (GnRH) agonist (for example, goserelin, 3.6 mg once every 4 weeks or 10.8 mg once every 12 weeks). The use of leuprolide and nafarelin has not been established for hormone reassignment therapy.
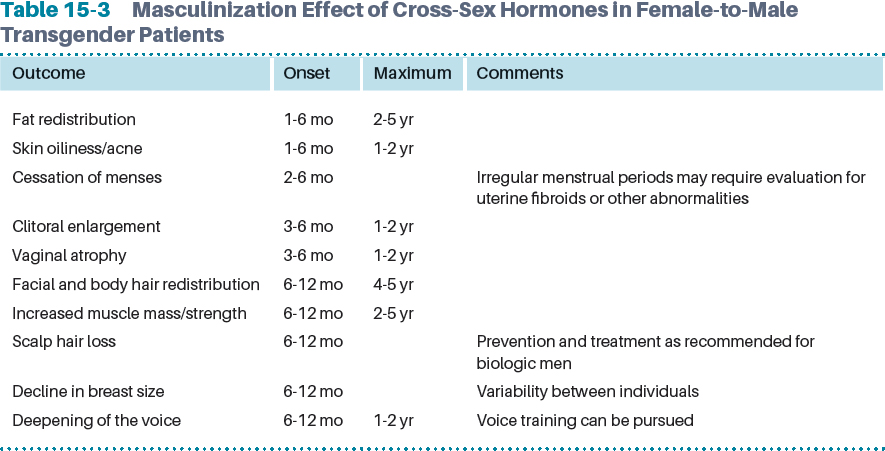
The estimated time for physical changes with testosterone therapy occurs in the first 3 to 6 months of treatment, although the maximum effect can take as long as 2 to 5 years for some patients8,14 (Table 15-3).
Routine Follow-up: Recommended Clinical Practice
The Endocrine Society recommends maintaining testosterone levels in the normal adult male range to prevent long-term side effects from therapy, such as erythrocytosis or transient elevation of liver enzymes. For this purpose, monitoring should be done every 3 months during the first years of therapy, and then once or twice a year thereafter8 (Table 15-4).
Table 15-4 Monitoring Female-to-Male Transgender Patients Receiving Hormone Therapy
Evaluation | Frequency | Comment |
History | Baseline | Specifically assess family history of diabetes, osteopenia, smoking, and previous bone fractures |
Physical examination (blood pressure, weight) | Baseline and every 2 to 3 mo in the first year and then yearly | Look for appropriate signs of masculinization and possible complications |
Serum total testosterone | Every 2 to 3 mo until levels are between 350 and 1000 ng/dl | Adjust dose |
|
| See Table 15-2 regarding when to measure based on preparation used |
Serum estradiol | Every 2 to 3 mo during first 6 mo or until cessation of menses | Usually serum estradiol <50 pg/ml in first 3 mo |
Liver function tests and CBC, lipid profile | Baseline; every 3 mo in the first year and then 1 to 2 times per year | Family history of diabetes should prompt A1c |
BMD measurement | Baseline and at 60 yr of age Yearly | If risk factors are identified |
Pap smear | Per American Cancer Society recommendations | If cervical tissue is present |
Mammogram |
|
|
Monitoring of weight, blood pressure, and physical changes, and asking routine health questions regarding new risk factors focusing on cardiovascular risk and new medications should be done at each visit. In addition, periodic monitoring of CBC, renal function, liver enzymes, and lipid and glucose profiles is also recommended.
Cardiovascular Risk
The metabolic effect of testosterone on the lipid profile is mainly on the increase of serum triglycerides and reduction of high-density lipoprotein levels15 with central fat redistribution for FTM patients. However, there is uncertainty regarding the degree of increase of cardiovascular risk with chronic testosterone use in FTM patients given the dearth of medical evidence in this matter. On the contrary, data from a meta-analysis of randomized clinical trials assessing the risks of adverse events associated with testosterone replacement in older men found no increased incidence of cardiovascular events in the treated group.16,17 Furthermore, data available from a university practice in The Netherlands with a median follow-up of 18½ years showed that for FTM transgender patients, total mortality and cause-specific mortality were not significantly different from those of the general population, and that for MTF transgender patients, the main increase in mortality was from non-hormone-related causes.18 Nevertheless, cardiovascular risk factors should be prevented and assessed according to available guidelines.19
Based on the available evidence, there is no increased risk of developing venous thromboembolism during cross-sex hormone treatment in FTM transgender patients receiving androgen treatment.20
Bone Health
Sex steroid exposure has been found to influence bone metabolism.21–23 Prior studies in FTM transgender patients have shown that in the first 2 years of hormonal sex reassignment therapy, testosterone administration could prevent bone loss resulting from estrogen deficiency.24 Cortical bone is the most affected by androgen replacement therapy, showing higher BMD. During the first year of cross-sex hormonal therapy with testosterone, there is an increase in bone turnover markers.25
At the molecular level, testosterone can affect bone physiology in an indirect or direct fashion. The use of exogenous testosterone lowers the receptor activator of nuclear factor kappa B ligand (RANKL) levels but does not change osteoprotegerin levels, resulting in an increased osteoprotegerin/RANKL ratio, which may be beneficial to the bone by inhibiting osteoclastogenesis. Furthermore, aromatization of testosterone to estradiol affects the bone directly by increasing BMD.26 In addition, chronic testosterone exposure has an impact on body composition by increasing muscle and decreasing fat mass. Moreover, there is a direct effect on the adult skeleton,25 with larger bone and lower volumetric BMD at the radius and tibia, in FTM transgender patients when compared with age-matched females.
Overall, the available evidence shows preserved BMD in the transgender population. Thus an adequate level of serum testosterone (300 to 1000 ng/dl) must be maintained to preserve the beneficial effect of androgen therapy in the bones. For this purpose, luteinizing hormone can be used as a marker of adequate hormone dosing, of which the goal is to keep its level in the normal range. This recommendation is based on the inverse correlation between luteinizing hormone or follicle-stimulating hormone and BMD.27 Sufficient intake of vitamin D and calcium initiated and maintained as indicated in the standard guidelines for the general population is recommended.
Cancer Screening
The effects of lifelong administration of testosterone therapy on cancer remain to be determined; however, some evidence indicates that prolonged exposure to androgen can lead to increased endogenous estrogen levels mostly by partial aromatization of testosterone to estradiol, which triggers endometrial hyperplasia and possible estrogen receptor–positive breast cancer. However, a large cohort study of adverse events on a transgender population showed no increase in hormone-related cancers.28
Ovarian Cancer
Three cases of ovarian cancer in FTM transgender patients have been reported.29,30 Immunohistochemical studies done in ovarian and endometrial tissue of FTM transgender patients receiving long-term treatment with testosterone therapy have shown an increase in androgen receptors, resembling those changes found in the ovarian tissue of patients with polycystic ovary syndrome,31 which may lead to androgen receptor–related ovarian cancer. To prevent the risk of female reproductive tract diseases and cancer, the Endocrine Society recommends total hysterectomy and oophorectomy for FTM transgender patients receiving cross-sex hormone therapy. Although a seemingly prudent recommendation, this is based on very low evidence of support.8
Cervical Cancer
Currently there are no data regarding the prevalence of cervical cancer or cervical cancer screening among FTM transgender patients. Yet the American College of Obstetricians and Gynecologists recommends that an FTM transgender patient who has not undergone hysterectomy should follow the same screening guidelines as nontransgender females (American Society for Colposcopy and Cervical Pathology guidelines: www.ascp.org/guidelines).
In brief, the recommendations are as follows:
 Women younger than 21 years of age should not be screened, regardless of age at sexual initiation and other behavior-related risk factors.
Women younger than 21 years of age should not be screened, regardless of age at sexual initiation and other behavior-related risk factors.
 Women 21 to 29 years of age should have a Papanicolaou (Pap) test every 3 years.
Women 21 to 29 years of age should have a Papanicolaou (Pap) test every 3 years.
 Women 30 to 65 years of age should have a Pap test and human papillomavirus test (co-testing) every 5 years (preferred); it is acceptable to have a Pap test alone every 3 years.
Women 30 to 65 years of age should have a Pap test and human papillomavirus test (co-testing) every 5 years (preferred); it is acceptable to have a Pap test alone every 3 years.
Breast Cancer
In one of largest studies in the transgender population, the estimated incidence of breast cancer in FTM transgender patients was significantly lower than in biologic women (5.9 per 100,000 person-years versus 154.7 per 100,000 person-years for biologic women).32 Today there are eight cases of breast cancer in the FTM transgender population published in the literature.32,33 This low incidence seems to be related to the high prevalence of mastectomy and testosterone therapy in the FTM transgender population. This estimation was based on a relatively small number of cases of breast cancer, and thus there should be cautious interpretation of these data.
The breast tissue of FTM transgender patients receiving long-term treatment with androgen therapy shows decreased glandular and increased fibrotic tissue. Based on the premise that exogenous testosterone is partially aromatized to estradiol and that the endogenous levels of estradiol do not decrease significantly in a treated FTM transgender patient, it is reasonable to link testosterone therapy with increased risk of breast cancer, especially in those patients who have not undergone total mastectomy. Conversely, breast cancer may develop in residual tissue after mastectomy. As a prevented intervention, a breast examination should be performed before initiation of cross-sex hormone therapy, with further assessment of family history for breast cancer. The Endocrine Society guidelines recommend following the same breast cancer screening guidelines for the general population.8,34
Male-to-Female Transgender Patients
Treatment Protocols
The hormone treatment needed to achieve phenotypic feminization of MTF transgender patients has two major components. The first is to decrease the virilization effect of endogenous testosterone with the use of antiandrogen agents, and the second is to promote feminization with estrogens. The main goal of using progestational agents (cyproterone acetate, not available in the United States), GnRH analogs (leuprolide, nafarelin, or histrelin), and other medications that suppress androgen action is to obtain a reduction in serum testosterone levels similar to the ones found in adult women (less than 55 ng/dl).
Table 15-5 Hormone Therapy Options for Female-to-Male Patients
Therapy | Dose | Comments |
Estrogen |
|
|
Oral estradiol | 2-8 mg/dl | Metabolized via the cytochrome P450 enzyme system; thus a potential drug-drug interaction can exist |
Transdermal patch estradiol | 0.1-0.4 mg twice weekly | Transdermal estradiol produces fewer changes in hemostatic variables |
Parenteral estradiol | 5-30 mg IM every 2 wk | Transdermal estradiol produces fewer changes in hemostatic variables |
Antiandrogen Therapy |
|
|
Progesterone | 20-60 mg PO daily | Progesterone affects lipid profile and BMD |
Spironolactone | 100-200 mg PO daily | Use off-label |
GnRH agonist (leuprolide) | 3.75-7.5 mg IM monthly |
|
Cyproterone acetate | 50-100 mg PO daily | Not available in the U.S. |
Finasteride | 1 mg PO daily |
|
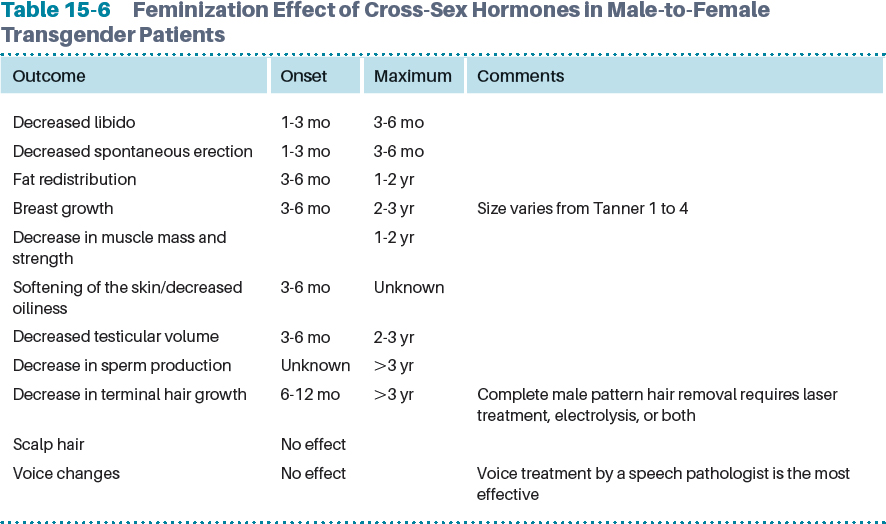
GnRH agonists are mostly used in adolescent patients in Europe. Testosterone secretion is suppressed by inhibiting the release of gonadotropins; this mirrors the effects achieved by bilateral gonadectomy, which reduces testosterone to minimal levels. The downside of these medications is the high cost, and that it is not covered by health insurance in the United States. A second, much less expensive drug, spironolactone, which inhibits testosterone secretion and androgen effects by inhibiting its binding to the androgen receptor, can be used. In addition, it has some weak estrogenic activity8 (Table 15-5). After orchiectomy is performed, antiandrogen therapy is no longer recommended or needed. Estrogen is available in oral, transdermal, and parenteral formulations as conjugated estrogens, 17 beta-estradiol, and estrogen ester.
Testosterone production is diminished by estrogen treatment by suppressing gonadotropin output; however, it is more effective when used in addition to other antiandrogen treatments. In general, estrogen should be used in conjunction with antiandrogen agents, especially in patients who have not undergone orchiectomy, because otherwise the doses needed to suppress testosterone levels to the minimal range and to maximize feminization will be four to eight times greater than in a biologic woman, increasing the risk for adverse events, namely, thromboembolic events.13 The Endocrine Society guidelines recommend keeping serum estradiol at the mean level for premenopausal woman (less than 200 pg/ml) and testosterone level lower than 55 ng/dl. These levels should be measured every 3 months after cross-hormonal therapy has been initiated.8