Adrenal Primary Tumors and Nontumors
Debra L. Zynger
ADRENAL CORTICAL ADENOMA, HYPERPLASIA, AND NODULES
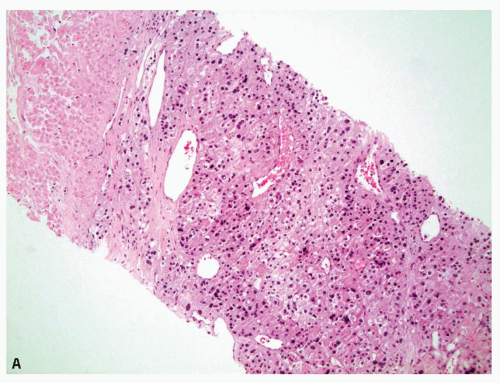
A nonneoplastic increase in the cellularity of the adrenal cortex is termed adrenal cortical hyperplasia. Adrenal cortical hyperplasia may be diffuse or produce multiple nodules. Hyperplasia is usually bilateral but can yield macronodules which mimic the appearance of an adrenal cortical adenoma. Adrenal cortical adenomas are typically unilateral, solitary, and well circumscribed. Adrenal cortical adenomas are thought to be a neoplastic process, supported by clonality in the majority of lesions, whereas most cases of nodular hyperplasia have been shown to be polyclonal, although there is overlap between the two diagnostic categories (1,2). Adrenal cortical hyperplasia and adrenal cortical adenoma can produce endocrine abnormalities or be nonfunctional. As there is a spectrum with morphologic and histologic overlap and from a practical perspective the lesions cannot be differentiated within a biopsy (and sometimes cannot be differentiated within a resection), these entities are discussed together. Additionally, an adrenal core biopsy cannot be used to distinguish normal adrenal cortex from an adrenal cortical adenoma or adrenal cortical hyperplasia (Fig. 10.1). This is significant, as the second most common diagnosis in adrenal core biopsies is adrenal cortical tissue (3). Therefore, the presence of benign adrenal cortical tissue does not ensure that the mass is a macronodule/adenoma and does not exclude an interventionalist sampling error.
Microscopic Features
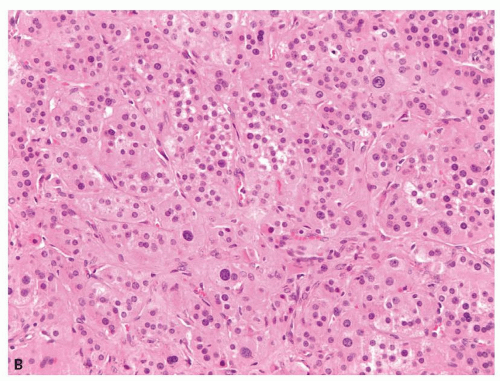
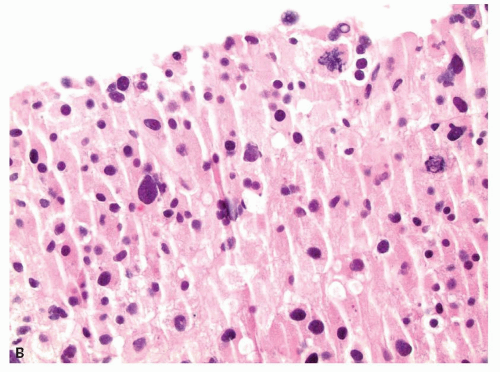
Small cortical nodules may be entirely confined to the cortex. Larger nodules may compress surrounding tissue and extrude into the adrenal capsule and periadrenal adipose tissue. Architectural patterns of growth that are common in nodules include nests, solid sheets, and trabeculae (Fig. 10.2). Rarely, pseudoglandular or myxoid features are present (Fig. 10.3). Cortical nodules consist predominately of finely vacuolated cytoplasm containing lipid droplets, similar to the zona fasciculata, but oncocytic cells can also be seen. The cells may be larger than normal adrenal cortex,
and cells with nucleomegaly and nuclear pseudoinclusions can be seen. Importantly, mitotic figures are quite rare and atypical mitotic figures are not present. Lipofuscin pigment may be identified. Uncommon findings include myelolipomatous, lipomatous, or osseous metaplasia (Fig. 10.4). Treatment with spironolactone for aldosterone-secreting adenomas may result in dense eosinophilic cytoplasmic inclusions, termed spironolactone bodies (Fig. 10.5). Degenerative changes including hemorrhage,
hyalinization, and calcification can be seen in larger nodules and should not be overinterpreted as characteristics concerning for malignancy. Adrenal cortex near the nodule may be hyperplastic in cases of macronodular hyperplasia, atrophic if the nodule is functional, or normal if the nodule is a nonfunctional adenoma.
and cells with nucleomegaly and nuclear pseudoinclusions can be seen. Importantly, mitotic figures are quite rare and atypical mitotic figures are not present. Lipofuscin pigment may be identified. Uncommon findings include myelolipomatous, lipomatous, or osseous metaplasia (Fig. 10.4). Treatment with spironolactone for aldosterone-secreting adenomas may result in dense eosinophilic cytoplasmic inclusions, termed spironolactone bodies (Fig. 10.5). Degenerative changes including hemorrhage,
hyalinization, and calcification can be seen in larger nodules and should not be overinterpreted as characteristics concerning for malignancy. Adrenal cortex near the nodule may be hyperplastic in cases of macronodular hyperplasia, atrophic if the nodule is functional, or normal if the nodule is a nonfunctional adenoma.
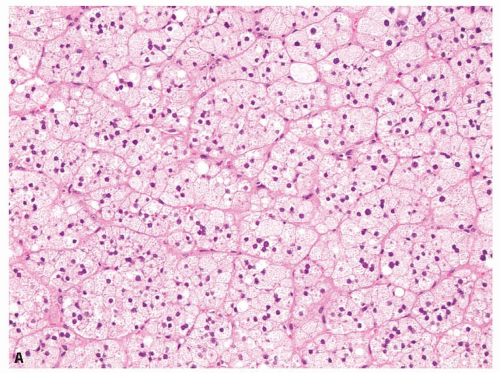 FIGURE 10.2 Adrenal cortical adenoma. A: Nests of finely vacuolated cells. B: Sheets of oncocytic cells with occasional nucleomegaly. |
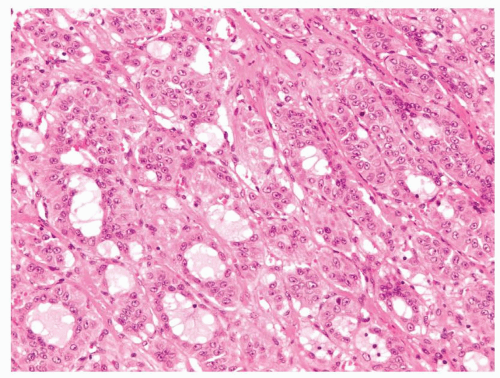 FIGURE 10.3 Pseudoglandular features are seen within this adrenal cortical adenoma which can mimic metastatic adenocarcinoma. |
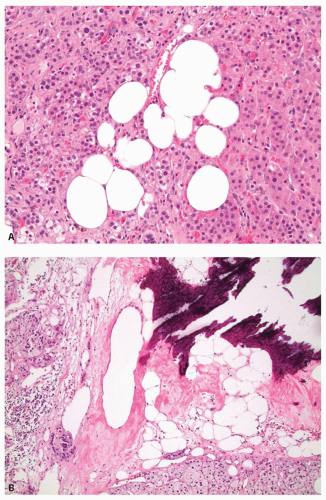 FIGURE 10.4 Unusual features in adrenal cortical adenomas. A: Lipomatous metaplasia. B: Osseous metaplasia. |
Ancillary Studies
Normal adrenal cortex, adrenal cortical adenoma, and hyperplastic adrenal cortex have consistent expression of inhibin alpha, calretinin, Melan-A, and steroidogenic factor-1 (SF-1) (4,5,6,7,8,9,10,11,12,13,14,15,16,17). Chromogranin A and S100 protein are negative (15,18,19). Carbonic anhydrase IX (CA-IX) has little to no membranous reactivity (4,20,21).
Differential Diagnosis
Low-grade clear cell renal cell carcinoma mimics the clear cells of adrenal cortex, and misinterpretation as clear cell renal cell carcinoma for adrenal cortical tissue in an adrenal core biopsy has been reported (3). Histologically, clear cell renal cell carcinoma has cells with more cleared out cytoplasm rather than the uniform fine vacuoles of adrenal cortical cells. As there is overlap and the cytoplasmic properties may not be appreciable on limited or poorly preserved biopsy material, immunostains can confirm the diagnosis with clear cell renal cell carcinoma negative using inhibin alpha, calretinin, Melan-A, and SF-1 but strongly positive in the cellular membranes using CA-IX (4,5,6).
Adrenal cortical adenoma cannot always be reliably histologically differentiated from adrenal cortical carcinoma, and this is especially difficult in an adrenal core biopsy. Criteria for malignancy are discussed in the following section. If necrosis or mitotic figures are seen, the possibility of malignancy should be raised. Unfortunately, ancillary studies are not helpful.
ADRENAL CORTICAL CARCINOMA
Adrenal cortical carcinoma is a very rare and aggressive adrenal neoplasm, and the diagnosis of adrenal cortical carcinoma in an adrenal core biopsy is given in less than 2% of cases (3). The peak incidence is in the fourth and fifth decades, and there is a female predominance (22,23,24,25,26,27). Five-year survival is 25% to 40% with hepatic, pulmonary, and nodal metastases common (23,24,25,26,27,28). Approximately half of tumors are hormonally functional causing Cushing syndrome and/or virilization (22,27,28). Adrenal cortical carcinomas are usually large (median 10 to 13 cm) (25,26,27). Weights over 100 g are worrisome for malignancy. Tumors that are greater than 6.5 cm and/or greater than 500 g are frequently malignant, but neither size nor weight are sufficient to predict malignancy, as small tumors have been reported to metastasize and large tumors can be benign (23,26,27,29,30).
Microscopic Features
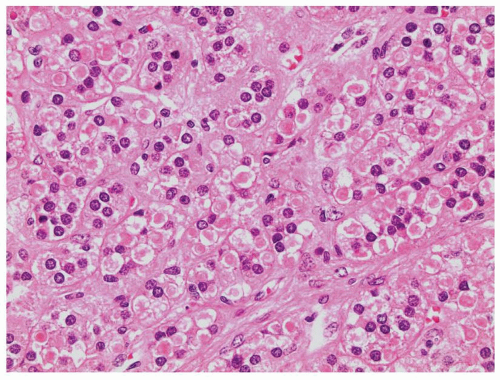
Adrenal cortical carcinoma consists of sheets, nests, and trabeculae of medium- to large-sized cells separated by sinusoids. The cytoplasm is eosinophilic to clear with vacuolization sometimes seen (Fig. 10.6). Cells may be monotonous with little nuclear atypia or demonstrate marked nuclear pleomorphism and hyperchromasia. Nuclear pseudoinclusions can be seen but are also seen in adrenal cortical adenomas. Necrosis, capsular invasion, and vascular invasion may be present (Fig. 10.7A). Mitoses vary from abundant to difficult to identify (Fig. 10.7B, eFig. 10.1). Approximately 10% of adrenocortical carcinoma will be composed of a predominance of large, oncocytic cells (31). Focal to diffuse myxoid stroma can be identified (Fig. 10.8). Spindling or sarcomatoid change is very rare. Cystic formation can occur in adrenal cortical carcinoma, and as such, a cyst should not be presumed to be benign (32,33,34). Other features that may rarely occur include lipomatous or myelolipomatous metaplasia, fibrosis, calcification, or metaplastic bone formation.
There are several histologic algorithms (Hough, Weiss, van Slooten, modified Weiss) to differentiate adrenal cortical carcinoma from adenoma (35,36,37). Mitotic rate is a key feature in these algorithms and has been shown to correlate with patient outcome (38). One such system is the modified Weiss criteria which are as follows: greater than 5 mitoses per 50 high-power fields, less than 25% clear cells, atypical mitotic figures, necrosis, and capsular invasion. The modified Weiss score is calculated as
follows: 1 point for the presence of atypical mitotic figures, necrosis, and capsular invasion and 2 points for the presence of greater than 5 mitoses per 50 high-power fields and less than 25% clear cells for a total score between 0 and 7. A score of greater than 3 highly correlates with subsequent malignant behavior (36). Certain modified Weiss features, specifically
the presence of clear cells, may not be applicable to eosinophilic adrenal neoplasms. Alternative algorithms have been proposed for oncocytic adrenocortical tumors (39,40).
follows: 1 point for the presence of atypical mitotic figures, necrosis, and capsular invasion and 2 points for the presence of greater than 5 mitoses per 50 high-power fields and less than 25% clear cells for a total score between 0 and 7. A score of greater than 3 highly correlates with subsequent malignant behavior (36). Certain modified Weiss features, specifically
the presence of clear cells, may not be applicable to eosinophilic adrenal neoplasms. Alternative algorithms have been proposed for oncocytic adrenocortical tumors (39,40).
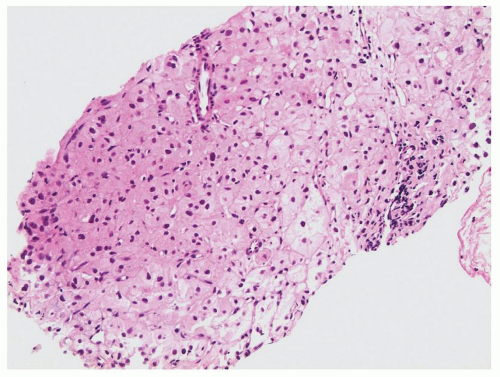
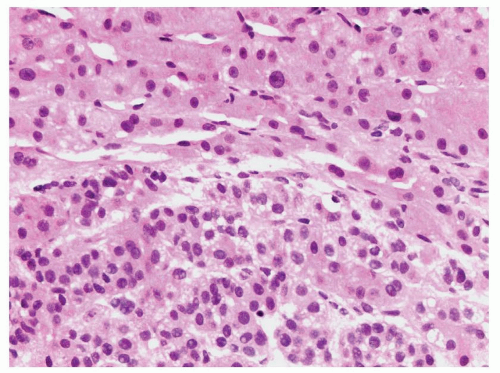 FIGURE 10.6 Adrenal cortical carcinoma (bottom half) in a liver biopsy (top half). The cytoplasm of the cortical carcinoma is finely vacuolated. |
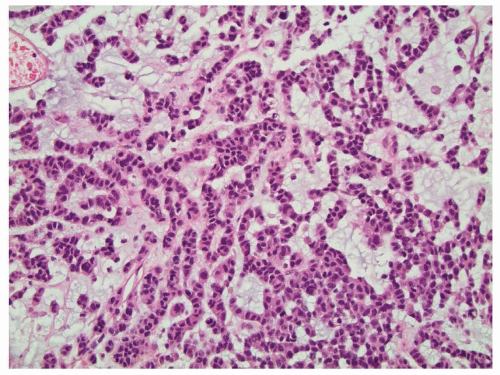 FIGURE 10.8 Myxoid material is seen in this adrenal cortical carcinoma which can mimic metastatic carcinoma. Liver metastases were present upon diagnosis. |
The features suggestive of malignancy described previously should be assessed in a needle core biopsy. As definitive diagnosis of malignancy may be difficult even in an adrenalectomy resection, it therefore may not be possible in a biopsy specimen unless there is a history of distant metastases (present at the time of diagnosis in 20% to 25% of patients) (eFig. 10.2) (25,26,27). If there is uncertainty regarding the diagnosis of malignancy, the surgical pathologist should describe the concerning histologic features and suggest the possible malignant potential of the lesion. This will be sufficient to proceed with a surgical resection which is the mainstay of treatment for adrenal cortical carcinoma (27,41).
Ancillary Studies
CAM5.2 is usually expressed although keratin reactivity can be weak (41). Inhibin alpha, calretinin, Melan-A, and SF-1 are highly expressed in the majority of adrenal cortical carcinomas (70% to 100%), similar to benign adrenal cortical lesions (4,5,6,7,8,9,10,11




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree