Adrenal Metastases
Debra L. Zynger
The most frequent entity encountered in an adrenal biopsy is metastatic carcinoma (1,2). Despite the small size, the adrenal is a common location for metastases. An adrenal biopsy in this clinical scenario is performed to stage and/or obtain diagnostic tissue. Adrenal core needle biopsy and fine needle aspiration have a high specificity for corroborating suspected metastasis to the adrenal gland (2,3,4,5,6). Sensitivity is lower, due in most part to sampling error (2). Although the majority of patients have a history of a nonadrenal primary tumor, the prior diagnosis of carcinoma may not be provided with the adrenal gland specimen (2). Additionally, unsuspected metastases have been detected in adrenal biopsy specimens (2,7).
METASTATIC LUNG CARCINOMA
In the first half of the 20th century, a large autopsy series identified breast (54%) as the most frequent carcinoma in adrenal metastases, followed by lung (36%) (8). In the latter half of the century, based on autopsies, adrenalectomies, and fine needle aspiration biopsies, the lung was found to be the most frequent metastatic carcinoma to the adrenal (35%), occurring at more than double the frequency of any other site, with the next most common sites being stomach, 14%; esophagus, 12%; and hepatobiliary, 11% (9). This change is reflected in adrenal biopsies, as lung carcinoma accounts for the majority of metastatic carcinoma encountered (46% to 75%) and is also the overall most frequent diagnosis (38%) rendered for these specimens (2,4,5,10,11).
The most important prognostic factor in lung non-small cell carcinoma is surgical resectability (12,13). Adrenal metastases are usually as-ymptomatic and occur in 2% to 3% of patients with otherwise resectable pulmonary non-small cell carcinoma (14,15). Imaging in this cohort there-fore must include the adrenal glands. If a suspicious lesion is detected in the adrenal gland, biopsy confirmation may be performed, particularly if the adrenal is the sole site of possible metastases. Diagnosing metastatic lung carcinoma has multiple roles in clinical management for these patients. If the lesional biopsy is found to contain adrenal cortex (nonneoplastic tissue/adrenal
cortical adenoma), staging is complete and the patient may undergo lung resection (14,15,16). For patients in which the adrenal is the only suspected site of metastatic spread, adrenalectomy should be considered, as with a complete surgical resection, a 5-year survival can be seen in 23% to 38% of patients (13,17,18,19). Approximately two-thirds of adrenal metastases are discovered synchronously with the lung primary and two-thirds are homolateral, with minimal difference in long-term survival based on timing of discovery or laterality of the adrenal tumor (13,19). An emerging reason to obtain tissue cores may be for molecular testing. In the past 2 years, 40% of our adrenal needle core specimens diagnosed with metastatic lung carcinoma have had molecular testing as a part of routine patient care, including analysis of KRAS, EGFR, and ALK, and rarely c-Met and ROS.
cortical adenoma), staging is complete and the patient may undergo lung resection (14,15,16). For patients in which the adrenal is the only suspected site of metastatic spread, adrenalectomy should be considered, as with a complete surgical resection, a 5-year survival can be seen in 23% to 38% of patients (13,17,18,19). Approximately two-thirds of adrenal metastases are discovered synchronously with the lung primary and two-thirds are homolateral, with minimal difference in long-term survival based on timing of discovery or laterality of the adrenal tumor (13,19). An emerging reason to obtain tissue cores may be for molecular testing. In the past 2 years, 40% of our adrenal needle core specimens diagnosed with metastatic lung carcinoma have had molecular testing as a part of routine patient care, including analysis of KRAS, EGFR, and ALK, and rarely c-Met and ROS.
Whereas in the past, a diagnosis of non-small cell carcinoma was sufficient, at this time, further classification using histologic and/or immunohistochemical expression is desirable whenever possible. In our experience over the last 5 years, the type of lung carcinoma present in adrenal core biopsies was 70% adenocarcinoma, 17% squamous cell carcinoma, 7% non-small cell (not further specified in either the adrenal core or the lung specimen if obtained), 3% small cell carcinoma, and 3% adenosquamous cell carcinoma (Fig. 11.1). This is similar to data from other published series from adrenal biopsies, adrenalectomies, and autopsy findings (4,9,13). The higher frequency of lung adenocarcinoma in adrenal biopsies compared to other histologic subtypes may be in part attributable to two factors. First, in the United States, adenocarcinoma is the most frequent type of lung carcinoma, and second, adenocarcinoma more commonly presents with distant disease relative to other lung histologies (20,21).
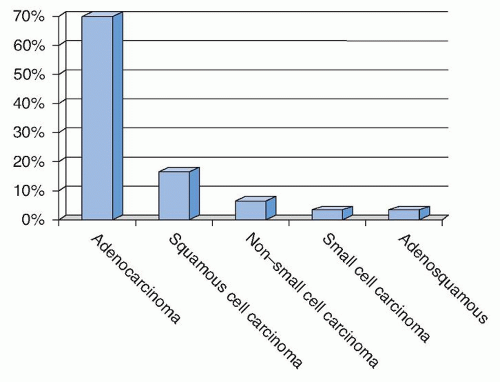 FIGURE 11.1 Types of lung carcinoma present within adrenal core biopsies from 2009 to 2013 at The Ohio State University Medical Center. |
Lung Adenocarcinoma
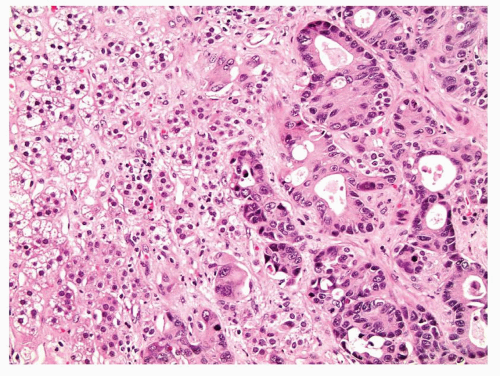
Lung adenocarcinoma is a malignant epithelial tumor composed of glands or cells with mucin production. These tumors may display a variety of growth patterns such as acinar or papillary, but within the adrenal metastasis these patterns may not be appreciable and are not relevant for clinical management (Figs. 11.2 and 11.3). The presence of malignant tumor with any suggestion of gland formation or mucin production within an adrenal biopsy should precipitate a workup to evaluate for metastatic carcinoma and specifically address lung adenocarcinoma if there is no prior history of carcinoma. Of note, adrenal cortical tumors may have a myxoid back-ground and pseudoglandular growth, mimicking adenocarcinoma (22,23,24).
Immunostains to be performed may include markers which are expressed in lung adenocarcinoma (CK7, TTF-1, and Napsin A), those expressed within adrenal cortical tumors (inhibin alpha, calretinin, Melan-A, steroidogenic factor-1 [SF-1] and synaptophysin), and markers to cover other entities within the differential diagnosis (broad spectrum keratin, CK20, etc). Nuclear expression of TTF-1 is seen in 60% to 96% of lung adenocarcinoma, whereas neither cytoplasmic nor nuclear reactivity has been reported in adrenal cortical carcinoma (eFig. 11.1) (25,26,27,28). Napsin A is positive in 80% to 90% of lung adenocarcinomas but also shows weak to moderate cytoplasmic staining in 10% of adrenal cortical tumors (eFig. 11.2) (29). Cytokeratin expression in adrenal cortical tumors is low (30,31). Inhibin alpha has reliable reactivity in adrenocortical tumors but also has variable expression in lung adenocarcinoma, with
some cases demonstrating diffuse positivity (32,33,34,35). Similarly, calretinin is reactive in adrenal cortical neoplasms, but approximately 10% of lung adenocarcinoma can be positive (36,37,38,39,40). Melan-A (clone A103) expression is consistent in adrenal cortical tumors with no reactivity in primary lung adenocarcinoma or lung carcinoma metastatic to the adrenal gland (41).
some cases demonstrating diffuse positivity (32,33,34,35). Similarly, calretinin is reactive in adrenal cortical neoplasms, but approximately 10% of lung adenocarcinoma can be positive (36,37,38,39,40). Melan-A (clone A103) expression is consistent in adrenal cortical tumors with no reactivity in primary lung adenocarcinoma or lung carcinoma metastatic to the adrenal gland (41).
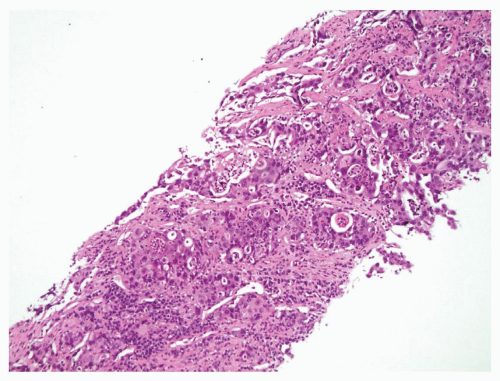 FIGURE 11.2 This adrenal core is almost entirely replaced with metastatic lung adenocarcinoma. Gland formation is evident. |
Lung Squamous Cell Carcinoma
Squamous cell carcinoma of the lung has histology comparable to other sites of the body. Well-differentiated squamous cell carcinoma will have very well-demarcated intercellular bridges and/or keratinization. In less differentiated tumors, these histologic features may not be present. There-fore, adrenal core biopsies containing metastatic squamous cell carcinoma originating from the lung may or may not have identifiable squamous cell features. Typically, the biopsy contains an infiltrate of nests of pleomorphic cells with moderate to abundant densely eosinophilic cytoplasm, brisk mitotic activity including atypical mitotic figures, macronucleoli, and prominent cell membranes (Figs. 11.4 and 11.5). Squamous pearls and keratinization are less common. Immunostains are helpful to resolve difficult cases. Almost all pulmonary squamous cell carcinoma is reactive with p63, although positivity can occasionally be seen in lung adenocar-cinoma (9% to 30%) and small cell carcinoma (31% to 77%) (eFig. 11.3) (42). CK5 or CK5/6 are consistently positive in pulmonary squamous cell carcinoma and are rare in lung adenocarcinoma; however, expression can be seen in adrenal cortical tumors (eFig. 11.4) (37,40,43,44). Regarding
adrenal markers, calretinin is positive in 40% of pulmonary squamous cell carcinoma, whereas inhibin alpha is not expressed (eFig. 11.5) (35,45). Importantly, squamous cell carcinoma from other locations should be considered, especially if there is no history of a lung tumor or in a patient with multiple lung nodules that might be metastases from another site as there is no way to histologically or immunohistochemically differentiate squamous cell carcinoma from different organs.
adrenal markers, calretinin is positive in 40% of pulmonary squamous cell carcinoma, whereas inhibin alpha is not expressed (eFig. 11.5) (35,45). Importantly, squamous cell carcinoma from other locations should be considered, especially if there is no history of a lung tumor or in a patient with multiple lung nodules that might be metastases from another site as there is no way to histologically or immunohistochemically differentiate squamous cell carcinoma from different organs.
 Get Clinical Tree app for offline access 
|