Adenocarcinoma
Incidence and Death Rates
The highest incidence rates for colon cancer occur in North America, Australia, and New Zealand, as discussed previously in this chapter. The risk for colorectal cancer rises significantly after age 40 in both men and women and doubles in each succeeding decade until age 75 (279). From 2000 to 2004, the median age at diagnosis for cancer of the colon and rectum was 71 years of age (2).
TABLE 14.14 Individuals at High Risk of Developing Colorectal Carcinoma | |
|---|---|
|
Approximately 1% were diagnosed between 20 and 34 years of age, 3.6% between 35 and 44, 11.1% between 45 and 54, 17.8% between 55 and 64, 25.7% between 65 and 74, 28.6% between 75 and 84, and 12.2% after 85 years of age. The incidence of a second primary colorectal malignancy increases in patients with one or more colorectal cancers (280). An increased colorectal cancer risk exists in the conditions listed in Table 14.14.
Family history is important in assessing colorectal cancer risk, especially in patients younger than 50 years of age. Genetic factors play a significant role in at least 20% of patients with colorectal cancer. Approximately 1% of the total cancer burden is contributed by FAP, 5% is associated with HNPCC, and 15% is contributed by other forms of familial cancer. HNPCC patients have an excess of proximal colonic cancers, while patients with FAP have predominantly left-sided cancers. FAP and HNPCC are discussed more extensively in Chapter 12.
Differences in the death rates from colorectal cancer relate to differences in socioeconomic factors, diet, population longevity, genetic factors, and the quality of available medical care (169). In the United States, death from colorectal carcinoma is uncommon before age 30 in the general population, but rises significantly after age 50. The overall 5-year relative survival rate for 1996–2003 from 17 SEER geographic areas was 64%. Five-year relative survival rates by race and sex were 64.9% for white
men, 64.9% for white women, 55.2% for black men, and 54.7% for black women (2). Colorectal cancer is declining in incidence and it is being detected at earlier stages of disease, leading to increased survival and decreased mortality rates.
men, 64.9% for white women, 55.2% for black men, and 54.7% for black women (2). Colorectal cancer is declining in incidence and it is being detected at earlier stages of disease, leading to increased survival and decreased mortality rates.
Sexual Differences
In North America and Australia (areas with high rates of colorectal cancer) and in Japan and Italy (countries with rapidly rising rates), the age-adjusted incidence of colorectal cancer
for men exceeds that for women. Women have higher rates of right-sided neoplasms and develop their cancers at an earlier age than men (281,282). Men exhibit a decline in the proportion of sigmoid cancers with an increase in transverse and descending colon cancer as they age. In some regions of the world, such as Southern Asia, rectal cancers occur more commonly in males, whereas colonic carcinomas affect both sexes equally (283).
for men exceeds that for women. Women have higher rates of right-sided neoplasms and develop their cancers at an earlier age than men (281,282). Men exhibit a decline in the proportion of sigmoid cancers with an increase in transverse and descending colon cancer as they age. In some regions of the world, such as Southern Asia, rectal cancers occur more commonly in males, whereas colonic carcinomas affect both sexes equally (283).
Racial Differences
In the United States, the highest colon cancer risk affects blacks, followed by whites. Intermediate levels exist in persons of Asian or Pacific Island descent. The lowest colorectal cancer risk is observed in Hispanics and Native Americans (2). A statistically significant percentage of black patients have proximally located lesions (284). Sixty percent of whites with colon cancers develop sigmoid cancer compared to 36.6% of blacks. Of all colonic cancers in blacks, 35% develop in the right colon, versus 24% in whites (284,285,286). The implication of this finding is that screening sigmoidoscopy may miss a significant proportion of colonic adenomas or carcinomas in black populations.
Location
Colon cancer risk varies by subsite within the colon (279). The location of colorectal carcinomas reflects screening practices, environmental and genetic factors, sexual and racial differences, and patient age. In low-risk countries, carcinomas of the cecum and ascending colon occur more frequently than carcinomas of the left colon, whereas in high-risk countries, colorectal carcinomas more commonly arise in the rectosigmoid region (169), a distribution similar to that seen in the United States (287). Right-sided colon carcinomas increase in incidence as patients age, particularly in women, with a progressive decline in the incidence of sigmoid and rectal lesions (285,288,289).
Multiple Tumors
Patients with colorectal cancer have a predisposition for developing more than one colorectal neoplasm, including both adenomas and carcinomas. In fact, the large bowel is
the organ most frequently involved by multiple primary malignant tumors (291,292,293,294,295,296). Patients with multiple tumors also have a greater chance of developing carcinoma in other organs of the body (293). Not surprisingly, such patients often have one of the hereditary forms of colon cancer. Multiple tumors occur commonly in patients with polyposis syndromes, HNPCC (see Chapter 12), and chronic IBD (see Chapter 11).
the organ most frequently involved by multiple primary malignant tumors (291,292,293,294,295,296). Patients with multiple tumors also have a greater chance of developing carcinoma in other organs of the body (293). Not surprisingly, such patients often have one of the hereditary forms of colon cancer. Multiple tumors occur commonly in patients with polyposis syndromes, HNPCC (see Chapter 12), and chronic IBD (see Chapter 11).
Multiple neoplasms may be synchronous (Fig. 14.79) or metachronous. Synchronous cancers are twice as frequent as metachronous ones (293,295). Synchronous neoplasms affect 35.9% of patients; 25.7% of the lesions are adenomas (294). The incidence rates of synchronous colorectal cancers range from 1.5% to 12% (292,293,294,295,296). Synchronous cancers often remain confined to the same region of the bowel, probably having arisen from clustered adenomas. However, since synchronous carcinomas can also be widely separated, a complete colonoscopy must be performed in every patient with colon cancer to search for additional tumors in other intestinal segments.
Metachronous tumors occur in 1.6% of patients with colorectal cancer (297). Patients with metachronous tumors tend to be somewhat younger when they originally present than patients with only single colon cancers; patients with synchronous lesions tend to be somewhat older (298). The incidence of metachronous tumors increases as the follow-up increases. Several large series, with prolonged follow-up, have reported intervals of 8.5 to 11 years between the development of the initial tumor and the subsequent development of a metachronous lesion (292,299,300). Up to 64% of patients have their second tumor discovered within 5 years of the first tumor, 45% within 3 years, and 20% within 1 year. The cumulative risk of a colon cancer survivor developing a metachronous growth increases from 3.5% after the resection of one cancer to 8% after the removal of two (299).
Clinical Features
Since colorectal carcinoma develops over a long period of time, it is not surprising that symptoms may be absent or are so slowly progressive that the patient fails to notice them. Thus, the patient may present with relatively minimal symptoms when diagnosed. From 5% to 12.5% of patients may remain asymptomatic (301,302). The clinical presentation depends on whether the tumors develop in the right or left colon and whether they are early or advanced (Fig. 14.80). Initial symptoms of colon carcinoma are usually vague and nonspecific. The symptoms and signs may relate directly to the gastrointestinal tract, or they may be constitutional in nature. Weight loss and malaise commonly occur, but these symptoms are often disregarded by the patient due to their nonspecificity. Cancers of the cecum and ascending colon are often flat or polypoid, and the stool is soft in this location. As a result, right-sided lesions often remain clinically “silent” because they fail to cause obstruction or visible melena. Weakness, malaise, fatigue, and weight loss in such patients may occur secondary to iron deficiency anemia. Cardiac failure or angina pectoris may be presenting symptoms in anemic patients.
Changes in bowel habits affect 22% to 58% of patients with colorectal carcinoma (302,303) and occur most frequently when the neoplasm arises in the left colon. The changes are often minimal, but progressive, and include diarrhea and a sensation of incomplete rectal emptying or incontinence. As the tumor grows and progressively encircles the bowel wall, the stool caliber decreases and constipation, obstipation, and other signs of bowel obstruction appear.
Rectal bleeding is the initial complaint in approximately 50% of patients (303). This nonspecific symptom can present as subtle degrees of bleeding, which may not be visible and are only manifested by the presence of an iron deficiency anemia. Frank hematochezia sometimes
develops. Rectal bleeding develops in about 70% of left-sided lesions, but often goes unnoticed by the patient. Fewer than 25% of patients with right-sided tumors notice any blood in the stools, probably because the blood becomes admixed with the feces. In a prospective study of patients presenting to general practitioners with a complaint of rectal bleeding, 10.3% of these patients had a colon cancer (304).
develops. Rectal bleeding develops in about 70% of left-sided lesions, but often goes unnoticed by the patient. Fewer than 25% of patients with right-sided tumors notice any blood in the stools, probably because the blood becomes admixed with the feces. In a prospective study of patients presenting to general practitioners with a complaint of rectal bleeding, 10.3% of these patients had a colon cancer (304).
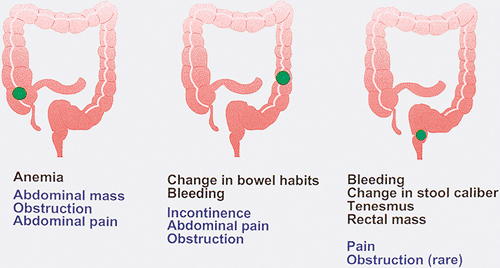 FIG. 14.80. The clinical symptoms that develop in patients with colon cancer depend on location of the tumor. Early symptoms are shown in black, and late symptoms are shown in blue. |
The issue of rectal bleeding is complicated these days by the use of NSAIDs, either to treat arthritic conditions or as prophylaxis for cardiovascular disease. These drugs are well known to cause intestinal bleeding. Rectal bleeding should never be attributed to NSAIDs, hemorrhoids, or rectal fissures, especially in older individuals, unless a malignancy has been ruled out. Some low-lying lesions are easily felt by a rectal examination (Fig. 14.81).
Abdominal pain is the presenting complaint in approximately 50% of patients with colon cancer. It is more likely to occur in patients with colon cancer rather than in those with rectal tumors (303). Pain often occurs in advanced colorectal cancer when the tumor invades the serosa (Fig. 14.82) or adjacent tissues. Occasionally, an obstructing, but not deeply penetrating, tumor may cause rupture of a diverticulum proximal to it. Lower abdominal pain is occasionally a symptom of cecal or ascending colonic lesions. Pain from left colonic lesions may result from varying degrees of bowel obstruction proximal to a constricting carcinoma (Fig. 14.83). Carcinomas at the ileocecal valve may mimic Crohn disease or cause appendicitis by obstructing the appendiceal lumen.
Approximately 20% of patients who present with colonic ischemia (so-called obstructive colitis) have a coexisting carcinoma causing the ischemia. Usually, the ischemic area develops proximal to the tumor and is separated from the tumor by a segment of normal-appearing colon. The tumor may also lie within the ischemic area (Fig. 14.84).
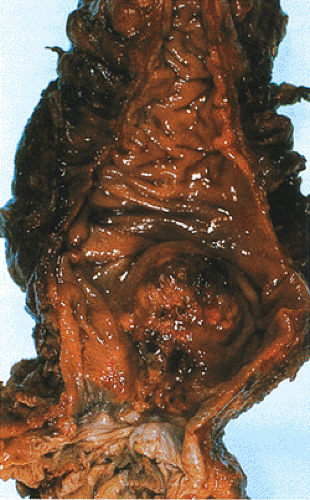 FIG. 14.81. Adenocarcinoma arising in the distal rectum just above the anal canal. This polypoid lesion was palpable on rectal examination. |
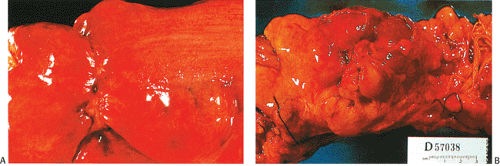 FIG. 14.82. Serosal aspect of the colon. A: A linear constricting lesion seen from the external surface of the bowel. B: There are large masses of tumor seen in external portions of the bowel. |
When a tumor invades through the bowel wall, a colo-colo fistula may develop (Fig. 14.85). Occasionally, a sigmoid carcinoma presents as an acute bowel obstruction or acute perforation with peritonitis. Peritonitis results from perforation of the bowel by a deeply invasive tumor or tumor extension into a diverticulum. Patients may also present with small bowel obstruction, particularly when the small bowel becomes adherent to, or invaded by, a colonic cancer.
Uncommonly, an otherwise clinically silent advanced carcinoma, most often involving the cecum, presents as an abdominal mass or hepatomegaly due to metastases. Unusual presentations of colon cancer include the development of gluteal abscesses or colocutaneous fistulae, fevers of unknown origin, or pyogenic arthritis.
Delays in the diagnosis of symptomatic patients fall into three major categories: (a) delays in scheduling initial or subsequent office visits or laboratory tests resulting in an average delay of 3 weeks (31% of delays), (b) physician-related delays resulting from a misdiagnosis or observation of symptoms without specific action (comprising 46% of all diagnostic delays and resulting in an average delay of 18 weeks), and (c) patient-related delays resulting in an average delay of 12 weeks (305). Children with colon cancers are especially likely to undergo a delay in diagnosis due to the failure to recognize the possibility of this disorder, especially if a family history for colorectal cancer does not exist.
Colorectal Cancers Arising in Polyposis Syndromes
Cancers developing in the polyposis syndromes are discussed in Chapter 12.
Colorectal Cancers Arising in Inflammatory Bowel Disease
Carcinomas developing in the setting of IBD are discussed in Chapter 11.
Colon Cancer in Young Patients
Colon cancer develops in <1% of individuals in the first 2 decades of life (306,307). Colon cancer has been found in a fetus, but the youngest living patient was 9 months old at the time of diagnosis (308). Young patients with colon cancer are likely to have FAP, HNPCC, another hereditary cancer syndrome (see Chapter 12), or IBD (see Chapter 11). However, occasionally colorectal cancer also develops in young patients without any predisposing known cause (306).
Symptom duration ranges from days to months. Tumors arising in the cecum usually have a shorter period of symptom duration (306). The nonspecific signs and symptoms resemble ordinary childhood abdominal diseases, including acute appendicitis (309,310). Abdominal pain and vomiting are usually present, but distension and the presence of a mass and a change in bowel habits are rare. Weight loss occurs late
in the disease. Anemia and visible evidence of bleeding almost exclusively associate with left-sided and rectal tumors (311).
in the disease. Anemia and visible evidence of bleeding almost exclusively associate with left-sided and rectal tumors (311).
It is common for young patients to experience a marked delay in their diagnosis due to their age. Therefore, they often have high-stage tumors at the time of diagnosis (306,307,308,312). The average delay between symptom onset and treatment is 6.5 months (313). Eighty-six percent of young patients have metastases at the time of diagnosis (314). Primary tumors arise throughout the large bowel. In one study, 25% of tumors involved the right colon, 24% the left colon, and 11% the rectum; 60% occurred in females (314). The predominant histologic type in children and adolescents is a poorly differentiated, mucin-producing carcinoma, contrasting with an incidence in adults of only 5% to 15% (309,310,311,312,315,316,317,318). A positive family history for colon cancer is present in 38% of patients.
Young patients appear to have a worse prognosis than their older counterparts, with 5-year survivals of 10% or less (309,315,319). The median overall survival is 4 to 8 months in those diagnosed late in the disease, compared with 24 months in patients who are diagnosed earlier (306). The most significant factor associated with survival is stage at presentation (307,313,314,318). Survival is also affected by the histologic type of tumor, tumor resectability, extent of bowel wall invasion, and presence of lymph node capsular invasion (314), which is an especially sensitive marker for short-term survival (307,314). Common sites of recurrence involve the ovaries and the omentum (307).
Colorectal Cancer Arising During Pregnancy
Colorectal carcinomas developing during pregnancy are extremely rare, estimated to affect <0.002% of pregnant women (320). Most carcinomas arise in the rectum and rectosigmoid (321). Predisposing factors, including FAP or IBD, are often present in this younger age group. Common complaints include abdominal pain and distension, nausea, vomiting, constipation, and bleeding (321). A delay in diagnosis is a significant problem, since the symptoms are often attributed both by the patient and the physician to the pregnancy itself. Complications include intestinal obstruction, bowel perforation, cancer perforation, and obstruction to the descent of the fetus by the tumor. Extensive metastatic lesions are often present at the time of surgery. No patient with colorectal carcinoma diagnosed during pregnancy reported in the literature has survived for more than 5 years. Choice of therapy depends on the operability of the tumor and the age of the fetus at the time of diagnosis.
Gross Features
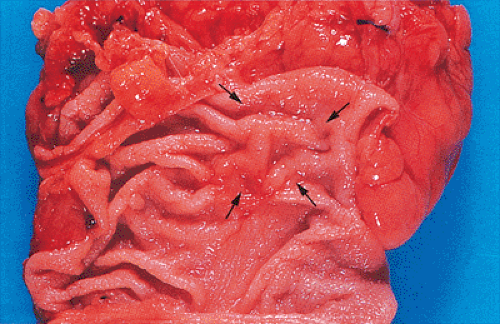
Small carcinomas measuring 1 to 2 cm in diameter are usually red, granular, buttonlike lesions that are variably elevated
above the tan mucosal surface. They are often sharply circumscribed, and grossly resemble adenomas (Fig. 14.79). Some are elevated only a few millimeters, whereas others are almost hemispherical in shape. Their consistency at this stage varies depending on the relative proportions of carcinoma, pre-existing adenoma, and the amount of coexisting stromal desmoplasia. As carcinoma replaces the adenoma, the tumor becomes firmer and paler (Fig. 14.79).
above the tan mucosal surface. They are often sharply circumscribed, and grossly resemble adenomas (Fig. 14.79). Some are elevated only a few millimeters, whereas others are almost hemispherical in shape. Their consistency at this stage varies depending on the relative proportions of carcinoma, pre-existing adenoma, and the amount of coexisting stromal desmoplasia. As carcinoma replaces the adenoma, the tumor becomes firmer and paler (Fig. 14.79).
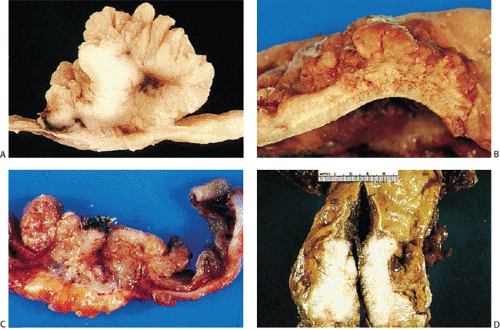
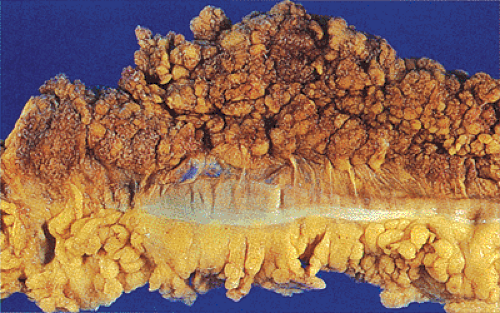 FIG. 14.86. Polypoid adenocarcinoma. A large exophytic tumor mass extends into the colonic lumen. The surface is lobular or papillary and demonstrates no evidence of ulceration. |
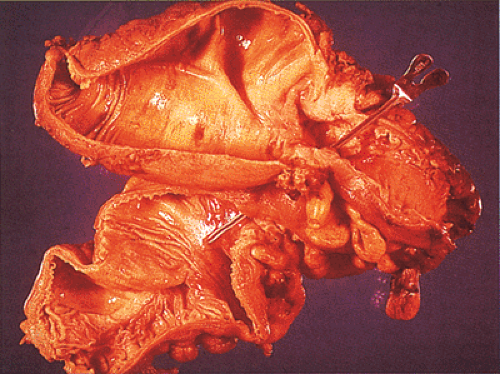
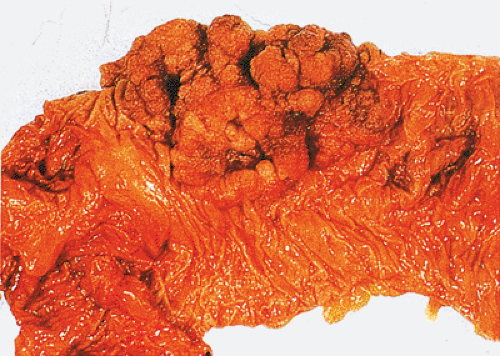
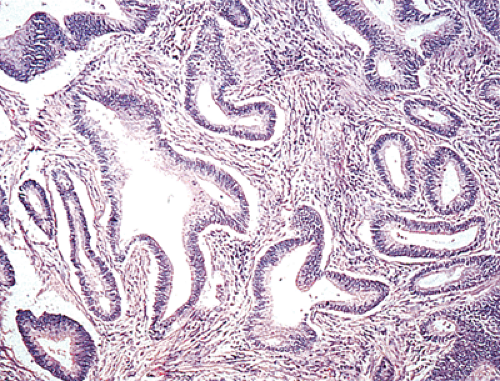
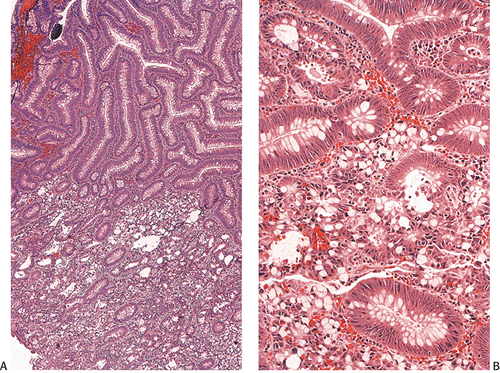
The gross appearance of colorectal carcinomas may be polypoid, fungating (exophytic), ulcerating, stenosing, or diffusely infiltrating. The polypoid type of carcinoma forms an exophytic intraluminal mass that has little in the way of surface ulceration (Fig. 14.86). It generally appears nodular, lobular, or papillary, and often contains residual adenoma. Approximately two thirds of all tumors are ulcerating; one third appear fungating. Bulky, fungating cancers often arise in the cecum and ascending colon (Fig. 14.87). Fungating lesions are basically papillary lesions with more ulceration. The ulceration destroys the underlying papillary architecture, leaving residual exophytic components. This type of tumor often has a raised or rolled border, and residual adenoma may be present. It tends to grow into the lumen and to extend along one wall, especially in the spacious cecum. Although it may occupy a large proportion of the colonic lumen, it rarely causes obstruction. The central part of these lesions typically feels firm, corresponding to the area of carcinoma. If soft areas are present, particularly at the edges of the lesion, this usually represents residual adenoma. This type of tumor often remains asymptomatic until blood loss results in anemia. The intraluminal tumor mass often exceeds the intramural tumor in volume (Fig. 14.88).
Ulcerating carcinomas (Fig. 14.89) deeply invade into the colonic wall. The edge of an infiltrating carcinoma is only slightly elevated above the surrounding normal mucosa, or it may be completely flat. The diffusely infiltrative type of carcinoma infiltrates a segment of the intestinal wall, often in a circumferential fashion, without forming a nodular mass.
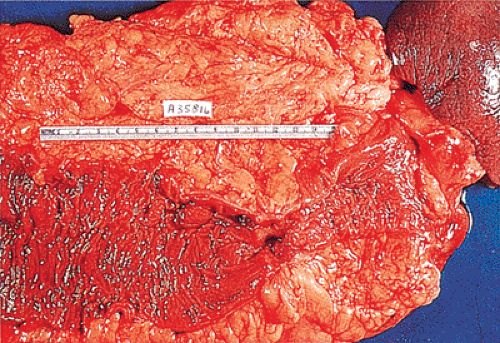
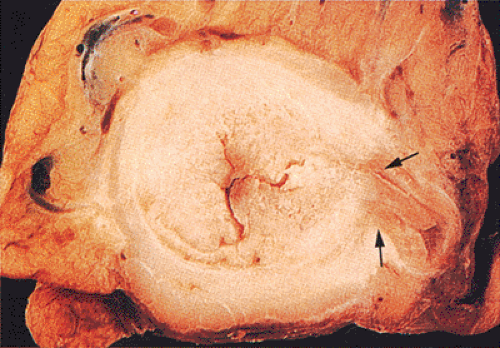
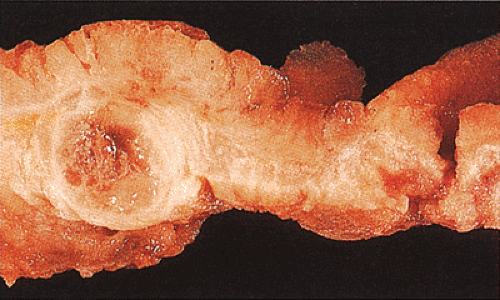
Adenocarcinomas arising in the transverse and descending colon usually become infiltrative and ulcerating, producing annular, constricting tumors (Fig. 14.90). They appear irregularly round, with raised, pale pink or white edges and central excavations (Fig. 14.83). They probably begin as locally infiltrative carcinomas that progressively encircle the bowel wall. These tumors obstruct the lumen and exhibit a
characteristic “apple core” or “napkin ring” appearance on barium contrast radiographs (Fig. 14.91). Except for their circumferential growth, their appearance resembles that of infiltrative carcinomas. Lateral intramural extension beyond the macroscopic border is unusual. The bowel usually dilates proximal to the tumor, with attenuation of the mucosal folds (Fig. 14.83). These tumors thicken the bowel wall and
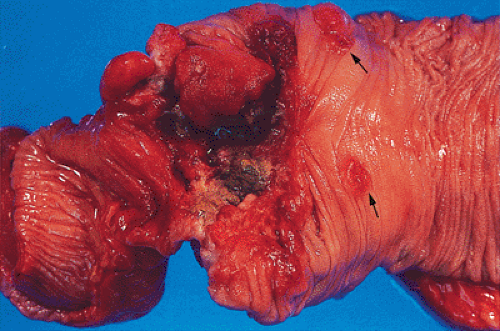
obliterate the muscularis propria, a feature best seen on the cut surface (Figs. 14.92 and 14.93). The tumors are firm due to the desmoplastic stromal reaction they induce. The intramural tumor volume may be at least as great as the luminal portion. When the tumors extend completely through the bowel wall, they may involve contiguous structures such as the small intestine, another part of the colon, or the stomach. Central necrosis and ulceration of a transmural tumor may cause perforation and peritonitis (Fig. 14.88). Combined or atypical growth patterns are not unusual; part of a tumor may be exophytic and the rest relatively flat (Fig. 14.92). Multilobed tumors suggest that close synchronous carcinomas coalesced (Fig. 14.94).
characteristic “apple core” or “napkin ring” appearance on barium contrast radiographs (Fig. 14.91). Except for their circumferential growth, their appearance resembles that of infiltrative carcinomas. Lateral intramural extension beyond the macroscopic border is unusual. The bowel usually dilates proximal to the tumor, with attenuation of the mucosal folds (Fig. 14.83). These tumors thicken the bowel wall and
obliterate the muscularis propria, a feature best seen on the cut surface (Figs. 14.92 and 14.93). The tumors are firm due to the desmoplastic stromal reaction they induce. The intramural tumor volume may be at least as great as the luminal portion. When the tumors extend completely through the bowel wall, they may involve contiguous structures such as the small intestine, another part of the colon, or the stomach. Central necrosis and ulceration of a transmural tumor may cause perforation and peritonitis (Fig. 14.88). Combined or atypical growth patterns are not unusual; part of a tumor may be exophytic and the rest relatively flat (Fig. 14.92). Multilobed tumors suggest that close synchronous carcinomas coalesced (Fig. 14.94).
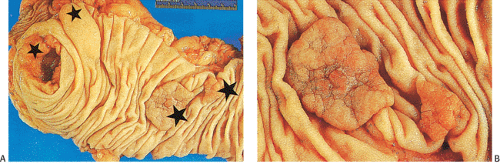
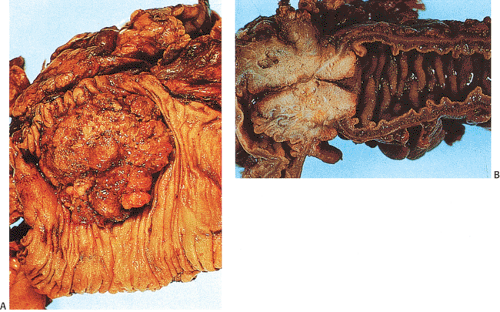 FIG. 14.87. Bulky carcinomas involving the cecum. A: A large exophytic lesion is present. B: The lesion almost completely blocks the cecum. |
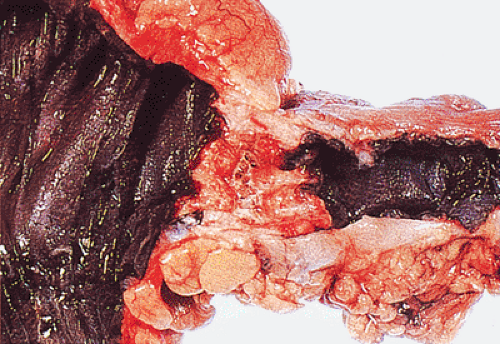
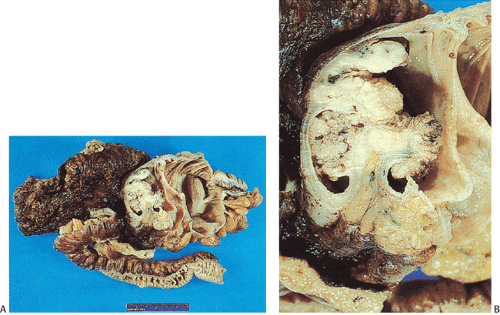 FIG. 14.88. Perforated adenocarcinoma of the colon. A: The entire colectomy specimen. B: Higher-power magnification showing the tumor itself. |
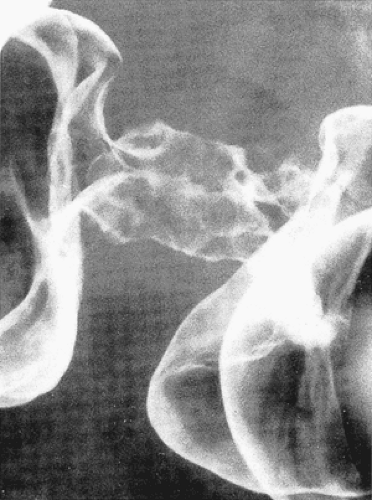 FIG. 14.91. Double-contrast enema demonstrates characteristic appearance of annular infiltrating adenocarcinoma. |
Diffuse infiltrating colorectal carcinomas are uncommon, but when they are present, they convert the colon into a rigid tube. This pattern of involvement resembles gastric linitis plastica. A fourth pattern of growth is the recently recognized flat or superficial carcinoma, which arises from flat adenomas. These carcinomas often appear as a flat plaque on the mucosal surface with extensive intramural invasion.
Histologic Features
General Comments
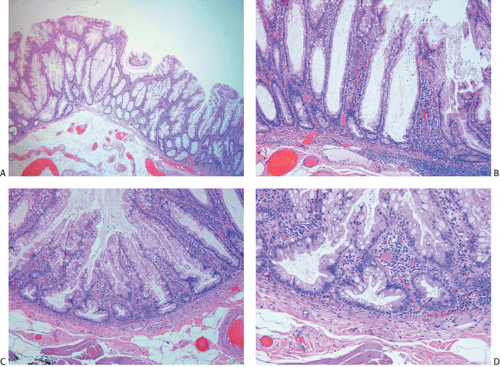
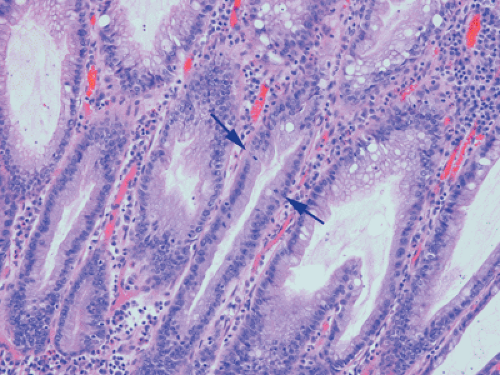
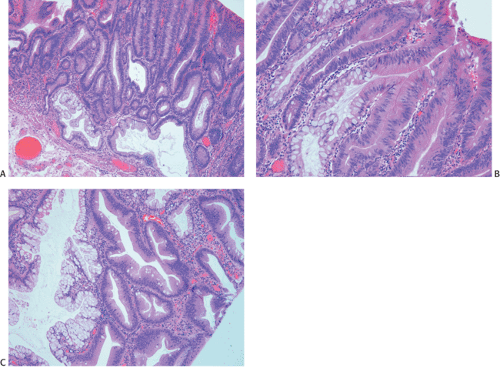
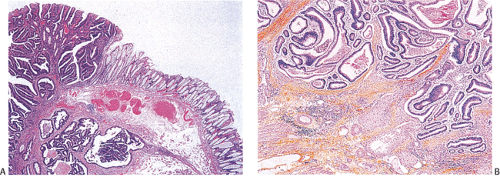
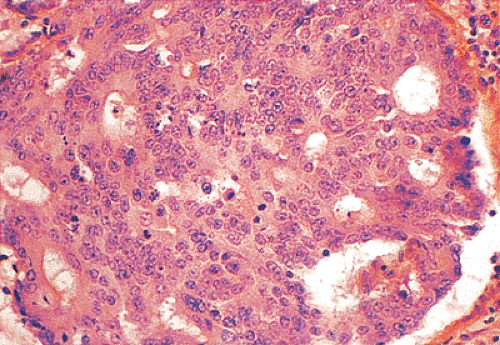
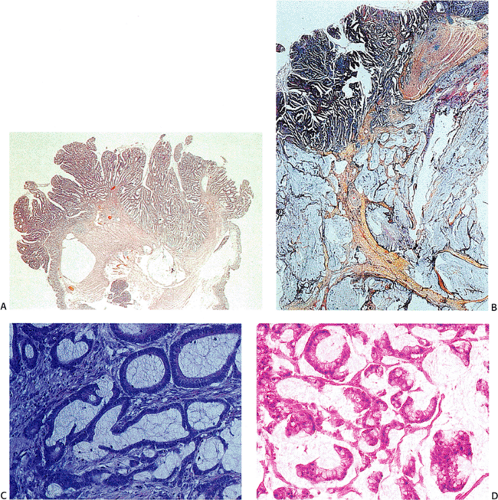
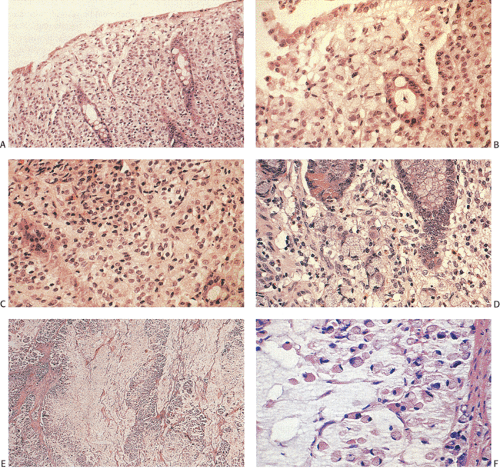
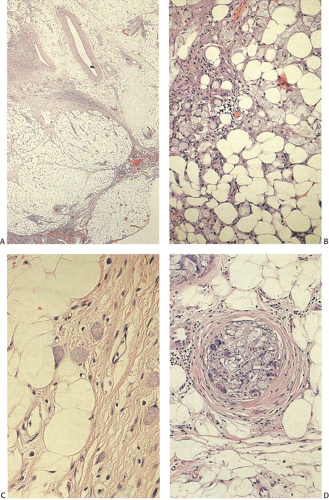
Ninety to ninety-five percent of all large bowel tumors are ordinary adenocarcinomas (322). They are usually easily recognizable as moderately to well-differentiated, gland-forming adenocarcinomas (Fig. 14.95). Twenty-five percent are well differentiated, 60% are moderately differentiated, and 15% are poorly differentiated (322). Tall, malignant, columnar cells with a high mitotic rate line large, irregular glands (Fig. 14.95). Well-differentiated carcinomas may demonstrate intraglandular papillary infoldings. The cells show cytologic anaplasia, although one may encounter malignant tumors that are so well differentiated that it is impossible to render a diagnosis of malignancy, except for the presence of glands infiltrating the bowel wall (Fig. 14.96). Even early invasive cancers usually induce a strong desmoplastic response. This aids in the diagnosis of minimally invasive carcinomas (Figs. 14.59 and 14.97). However, there are some colorectal carcinomas that invade the bowel wall without inducing much of a desmoplastic response. Such tumors may cause a diagnostic problem, particularly if the patient has associated diverticulosis. In such cases, it may be impossible to tell whether the lesion is invasive or not. In many cases, there is no histologic difference between the superficial portion of the tumor and deeply invasive or metastatic tumor. In other cancers, the deeper tumor may differ histologically from the surface. Mucin production ranges from virtually none to tumors producing so much mucin that they are designated as mucinous carcinomas. Many exophytic carcinomas exhibit a papillary structure composed of histologically malignant cells. However, the infiltrating intramural component is usually less obviously papillary, exhibiting moderately to well-differentiated glandular structures (Fig. 14.95). Rare tumors have a prominent papillary component even in their invasive parts (Fig. 14.96).
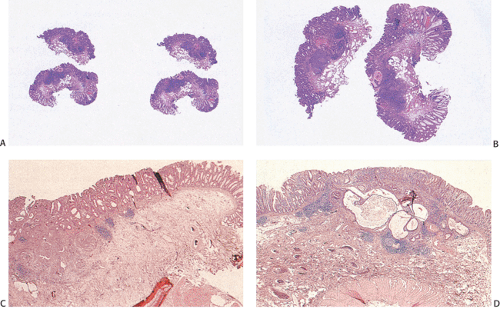
Residual adenomatous mucosa may be present at the edge of a malignancy, especially in smaller tumors. The majority of small carcinomas associated with residual adenomas are well differentiated. Some small polypoid carcinomas lack residual adenoma. These are only superficially invasive and are designated as polypoid carcinomas (Fig. 14.98).
One may also see hyperplastic glands adjacent to neoplasms. The mucosa appears taller and more tortuous than normal, with an increase in the number of goblet cells. This is probably a reactive change that occurs in response to mucosal abnormalities and is termed transitional mucosa. This mucosa differs histochemically from the surrounding normal mucosa (323).
Occasional colorectal adenocarcinomas mimic urinary bladder villous adenomas. Distinguishing a primary bladder adenocarcinoma from spread of a colonic carcinoma to the bladder may be impossible without consideration of factors other than the histology (324). Asbestos workers who develop colonic carcinomas may show the presence of asbestos bodies within the colonic tissue as well as in the mesentery (68).
Colonic adenocarcinomas invade through the wall, either in an expanding or an infiltrative growth pattern. Approximately 75% of tumors have relatively well-circumscribed, advancing margins, but 25% are more diffusely infiltrative. The expanding pattern consists of
nodular aggregates of neoplastic glands. Infiltrative tumors consist of individual cells or small glands infiltrating through the wall. Infiltrative tumors generally lack an inflammatory response, whereas this reaction is present in the expanding lesions. Expanding carcinomas usually exhibit a polypoid or fungating growth pattern, whereas infiltrative carcinomas are usually ulcerative or diffusely infiltrative.
nodular aggregates of neoplastic glands. Infiltrative tumors consist of individual cells or small glands infiltrating through the wall. Infiltrative tumors generally lack an inflammatory response, whereas this reaction is present in the expanding lesions. Expanding carcinomas usually exhibit a polypoid or fungating growth pattern, whereas infiltrative carcinomas are usually ulcerative or diffusely infiltrative.
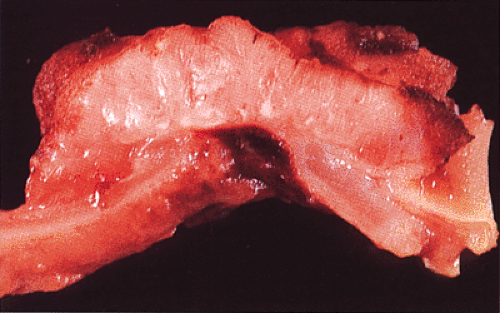 FIG. 14.93. Cut section through a colonic adenocarcinoma. The tumor obliterates the muscularis propria and invades to the serosal surface. |
Histologic Grading
Histologic grading is a routine practice in the pathologic reporting of large bowel cancer and is used as a prognostic indicator. Histologic grading is mainly gauged on a tumor’s architectural features. Well-formed glands are present in >75% of the tumors that are well differentiated, in 25% to 75% of moderately differentiated tumors, and in <25% of poorly differentiated carcinomas. In well-differentiated carcinomas, well-formed glands are lined by cells that maintain their nuclear polarity (Fig. 14.95). The glands resemble those present in adenomas. Poorly differentiated tumors predominantly consist of solid tumors composed of cells showing a loss of nuclear polarity and considerable nuclear pleomorphism (Fig. 14.99). Moderately differentiated tumors fall in between these two extremes (Fig. 14.100). Occasionally, one portion of a tumor appears well differentiated while another area is poorly differentiated (Fig. 14.101). The histologic grade is assigned according to the least differentiated area found, even though this may appear to be quantitatively insignificant. Tumor giant cells are sometimes present (Fig. 14.102). The cells in the solid areas may be completely anaplastic, or they may exhibit a signet ring morphology (Fig. 14.102).
Occasionally, one observes totally undifferentiated carcinomas that consist of large sheets of malignant cells. They contain abundant cytoplasm and minimal mucinous differentiation and, therefore, require further analysis to determine their epithelial nature. This can be confirmed by the use of immunostains for cytokeratin.
The agreement between the grade of a biopsy and corresponding resected tumor varies from 52% to 69%. Only 52% of poorly differentiated tumors are diagnosed as such in a preoperative biopsy. The poor predictive value is not improved by taking multiple biopsies (325). Thus, the grade
of colorectal carcinoma cannot be accurately assessed on a preoperative biopsy.
of colorectal carcinoma cannot be accurately assessed on a preoperative biopsy.
Cell Types in Carcinomas
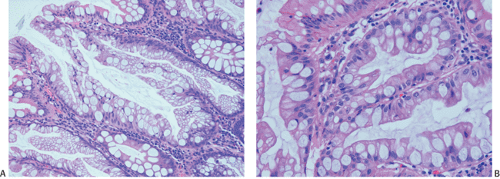
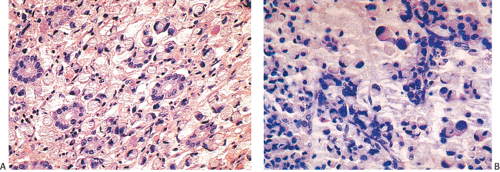
A number of cell types are present in colorectal adenocarcinomas. These include variably differentiated enterocytes, goblet cells, Paneth cells, endocrine cells (Fig. 14.103), squamous cells, melanocytes, and trophoblastic cells. Multipotential crypt stem cells can differentiate into these, and perhaps other cell types, thereby producing colorectal carcinomas that exhibit a number of distinctive histologic patterns. Some tumors are rich in Paneth cells, especially papillary carcinomas and mucinous tumors (326,327,328). The round, supranuclear granules of both normal and neoplastic Paneth cells stain red with hematoxylin and eosin stains. The Paneth cells lie interspersed singly or in small groups among the goblet cells. The Paneth cells are not mitotically active, but they do show obvious signs of neoplasia, including loss of polarity, cellular anaplasia, and an increased nuclear:cytoplasmic ratio. There is also marked variation in the number, shape, and electron density of Paneth cell granules. The presence of these cells has no impact on the biology of the tumor.
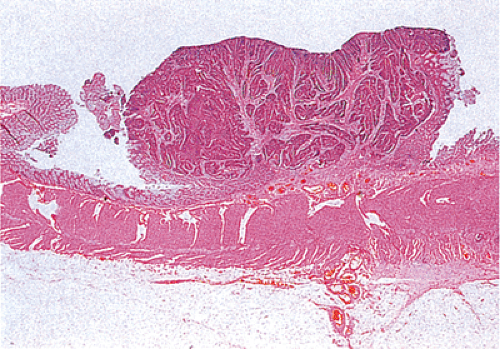 FIG. 14.98. Polypoid carcinoma. An exophytic tumor without residual adenoma of the epithelium is seen. |
Neuroendocrine cells are present in 8% to 51% of colorectal carcinomas (329,330,331,332). The endocrine cells usually appear at the edges of glands and may have clear or granular eosinophilic cytoplasm, allowing their histologic recognition. Their presence can also be confirmed using special stains or immunostains. Some authors indicate that there is no prognostic significance of neuroendocrine differentiation in colorectal adenocarcinomas (327,328), but others do not share this view (332,333,334,335,336).
Stromal Components
The stroma of colorectal carcinomas has largely been ignored, but the recent introduction of peritumoral lymphocytic
infiltration into the Jass (337) staging system calls attention to the need to evaluate this feature. The stromal component of colonic carcinomas varies from little or no stroma (see Fig. 14.108) to frankly scirrhous tumors (Fig. 14.104). Stromal fibroblasts actively proliferate. The activated fibroblasts produce collagen and other connective tissue proteins that induce the desmoplastic reaction. Prominent stromal elastosis surrounds some invasive carcinomas; a conspicuous proliferation of elastic fibers is also present in the vascular media of blood vessels lying in proximity to tumor cells (338).
infiltration into the Jass (337) staging system calls attention to the need to evaluate this feature. The stromal component of colonic carcinomas varies from little or no stroma (see Fig. 14.108) to frankly scirrhous tumors (Fig. 14.104). Stromal fibroblasts actively proliferate. The activated fibroblasts produce collagen and other connective tissue proteins that induce the desmoplastic reaction. Prominent stromal elastosis surrounds some invasive carcinomas; a conspicuous proliferation of elastic fibers is also present in the vascular media of blood vessels lying in proximity to tumor cells (338).
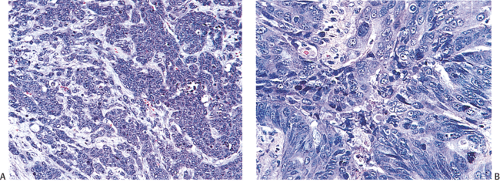 FIG. 14.99. Poorly differentiated adenocarcinoma. A: Irregular nests of tumor cells without obvious glandular differentiation. B: The cells are large and highly anaplastic. |
Colorectal carcinomas may also exhibit a peritumoral lymphocytic infiltrate, which can be judged as conspicuous or inconspicuous using the Jass criteria (337,339). These infiltrates consist of mast cells, eosinophils, macrophages, lymphocytes, plasma cells, and S100 protein–positive dendritic cells. Cytotoxic T lymphocytes constitute the majority of the infiltrating cells. The costimulatory molecules B7.1 (CD80) and B7.2 (CD86) by macrophages along the invasive margin of colon cancers is in accordance with the clinicopathologic studies that suggest that peritumoral lymphocytic infiltrates have a favorable prognostic factor. The presence of these cellular infiltrates suggests that there is an ongoing immune response potentially directed against the tumor cells.
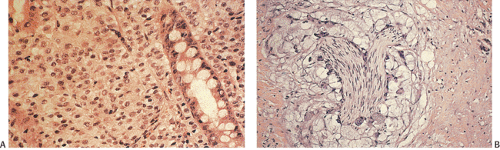
Another type of reaction developing around tumors is a Crohn-like reaction (Fig. 14.105). It is characterized by the presence of discrete lymphoid aggregates, often with germinal centers ringing the periphery of invasive carcinoma. It is typically found at the interface of the muscularis propria and pericolic fibroadipose tissue. The magnitude of the Crohn-like reaction correlates with patient survival (340).
Tumor Vessels
Neovascularization of colorectal carcinomas results in a disorganized vascular pattern of nodular capillary clusters and capillary sheets of frequently interanastomosing vessels. They may also almost completely pack the interstitial spaces, making the overall vascular volume of carcinomas greater than that of adenomas or the normal colon. These changes
are focal and occur even in intramucosal carcinoma. Most capillaries have a long tortuous course and numerous capillary sprouts. Tumor microvessel diameters are greater than normal (341).
are focal and occur even in intramucosal carcinoma. Most capillaries have a long tortuous course and numerous capillary sprouts. Tumor microvessel diameters are greater than normal (341).
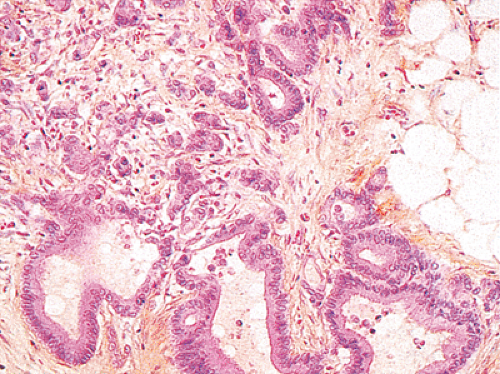 FIG. 14.101. An invasive component of this carcinoma is both well differentiated and poorly differentiated. |
The intratumoral blood vessels display various structural abnormalities, including endothelial cell proliferation, endothelial fenestrations, multilayered basement membranes, and thickened perivascular tissues. These features may result from repeated damage or may represent the effects of vascular remodeling (341). Red blood cells within the vessels also appear morphologically abnormal (341), perhaps explaining the propensity of colon cancers to form thrombi and produce the ischemia sometimes associated with them. The abnormal vessels may also facilitate intravascular invasion by the neoplasm.
Cancer Arising in Diverticula
Because colon cancer and diverticulosis often coexist in a specimen, the carcinomas may extend down into the diverticula (Fig. 14.106). When the orifice of a diverticulum is blocked by the tumor, diverticulitis may develop. Carcinomas may also develop within the diverticulum itself. Staging of such lesions poses problems. Because of the increased penetration of the wall inherent in the diverticular location, a conservative approach would mandate that one stage such lesions at the maximum level of extension in the bowel wall, even if it is in the diverticulum. The reason for making this statement is that virtually nothing is known about the subsequent biology of these lesions, and as a result, one should probably be conservative in the approach to this problem.
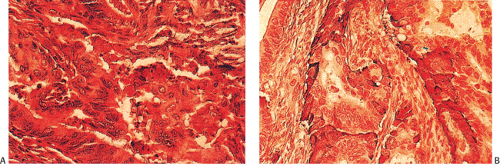 FIG. 14.103. Endocrine differentiation. A: A typical adenocarcinoma containing large numbers of endocrine cells. B: Grimelius stain demonstrates the presence of numerous endocrine cells. |
Pathologic Characteristics of Sporadic Colorectal Cancers with Microsatellite Instability
Microsatellite unstable (MSI) sporadic colon cancers differ phenotypically from microsatellite stable (MSS) tumors. In addition, the prognosis of MSI sporadic cancers may differ
from that of MSS neoplasms (342,343,344,345,346,347), making their recognition of potential importance in patient management. Patients with sporadic MSI cancers tend to be somewhat younger than other patients (60 6 5 years, range 22 to 83, vs. 66 6 1, range 27 to 90). MSI tumors have a marked tendency to develop proximal to the splenic flexure (94% vs. 34%). In comparison to MSS proximal tumors, MSI tumors have more frequent exophytic growth and large size, poor differentiation, extracellular mucin, a Crohn-like lymphoid reaction, and a trend toward less frequent p53 product overexpression by immunohistochemistry (348,349).
from that of MSS neoplasms (342,343,344,345,346,347), making their recognition of potential importance in patient management. Patients with sporadic MSI cancers tend to be somewhat younger than other patients (60 6 5 years, range 22 to 83, vs. 66 6 1, range 27 to 90). MSI tumors have a marked tendency to develop proximal to the splenic flexure (94% vs. 34%). In comparison to MSS proximal tumors, MSI tumors have more frequent exophytic growth and large size, poor differentiation, extracellular mucin, a Crohn-like lymphoid reaction, and a trend toward less frequent p53 product overexpression by immunohistochemistry (348,349).
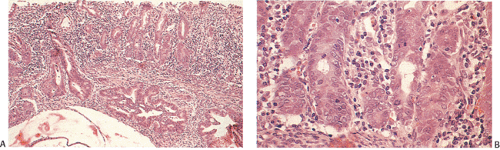
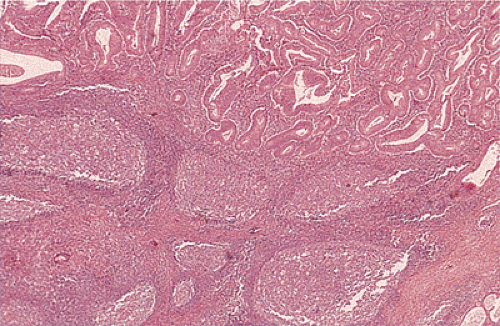 FIG. 14.105. Colonic adenocarcinoma with a marked Crohn-like tissue reaction. Well-formed lymphoid aggregates with prominent germinal centers lie at the margins of the tumor. |
Mucinous differentiation (>30% of the tumor) occurs in 39% to 75% of adenocarcinomas with an MSI phenotype and only 19% of MSS adenocarcinomas (348,349,350). Also, the plexiform pattern of the growth of the carcinoma significantly associates with the MSI phenotype (349). The plexiform pattern of growth is characterized by richly anastomosing and branching glandular spaces or cords in well- and poorly differentiated cancers, respectively (349). The plexiform pattern (irregular tubular differentiation) represents an early step of mucinous differentiation and should not be considered independent of it.
Flat Carcinomas
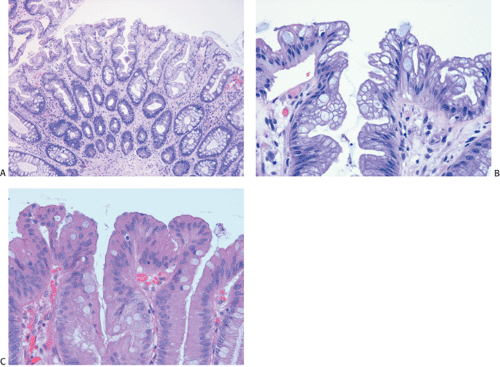
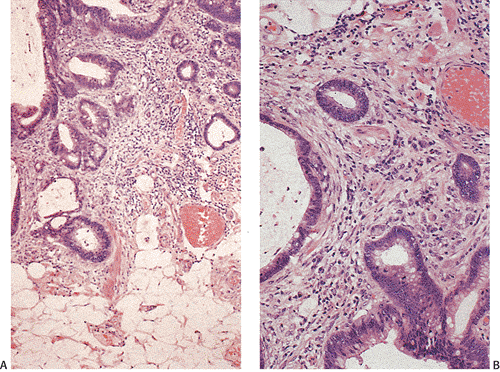
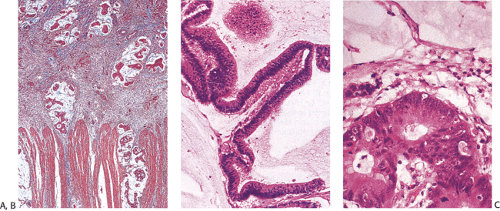
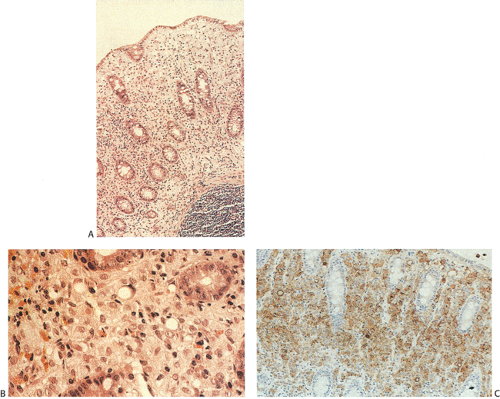
Flat carcinomas consist of slightly elevated, flat, or depressed lesions. This lesion is not rare in Japan, where it was first described, although it is rare in Europe and North America. Flat adenomas and adenocarcinomas resemble one another endoscopically and grossly. Softness of the intestinal wall and flabbiness of the tumor during endoscopy are clues to diagnosing adenoma endoscopically, whereas an endoscopic feature that suggests a cancer is stiffness of the tumor (212). Early cancers of the superficial type may appear nonpolypoid, flat, or depressed (Fig. 14.107). The height of the lesion does not exceed 50% of the tumor diameter (212,351). The mean size of small, flat colorectal cancers ranges from 1 to 20 mm with a mean of 6.7 mm, and is significantly smaller than polypoid lesions. When compared to polypoid lesions, flat colorectal cancers are more often proximally located, are less frequently well differentiated, and have fewer adenomatous remnants.
Controversy continues to surround superficial lesions, since disagreement exists concerning whether they represent true invasive carcinomas or areas of flat intramucosal carcinoma. Nonetheless, small carcinomas with clear-cut submucosal invasion do exist (Fig. 14.108). By the time they are detected, superficial adenocarcinomas may overgrow their associated adenomatous tissue (352), causing the tumor to resemble de novo carcinoma (351,353). Residual adenoma is present in only 8.1% of lesions. Only 40% of flat adenomas with a diameter <10 mm remain confined to the mucosa (212). The invasive tumors tend to be well or moderately differentiated.
Carcinomas that arise in flat adenomas develop from the base of lesions and may appear solid and/or cystic (Figs. 14.108 and 14.109). Epithelial cells in the basal parts of the crypts immediately adjacent to lymphocytic aggregates frequently show decreased amounts of mucus and cytoplasmic basophilia as well as increased nuclear:cytoplasmic ratios and mitotic figures suggesting active cellular proliferation (352). Even though superficial cancers invade only into the submucosa, they still may metastasize to the regional lymph nodes. Cancers invading the submucosa frequently develop lymphatic and/or vascular permeation. Lymphatic and vascular permeation occurs even when the lesions are small and may be more prevalent away from the rectosigmoid.
One rare form of flat carcinoma presents as an inverted, transmural, solid, and cystic lesion covered by a flat adenoma. The tumor may appear well differentiated, extending into the serosa and demonstrating a lobulated topography in the absence of a desmoplastic inflammatory stromal reaction. This pattern has been termed endophytic malignant transformation (354). Such transformation must be distinguished from the misplaced glandular epithelium as seen in localized colitis cystica profunda (354). Follicular lymphocytic aggregates frequently are observed in superficial-type adenocarcinomas but rarely in superficial adenomas (352).
Carcinomas Associated with Schistosomiasis
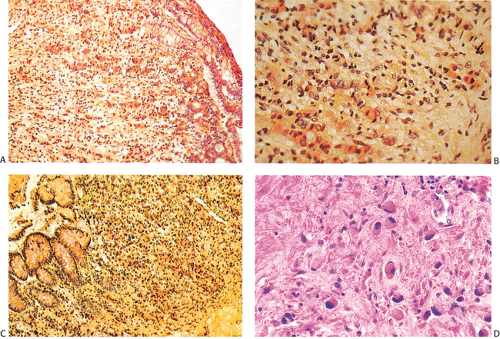
Carcinomas arising in the setting of schistosomiasis (S. japonicum) often arise on a background of granulomatous colitis with mild to severe dysplasia. The dysplasia is either focal or diffuse in its distribution and occurs in flat mucosa, pseudopolyps, or regenerating epithelium at the edges of ulcers. These dysplastic changes represent the pathologic basis for the future malignant development of schistosomal colitis and resemble the changes found in longstanding chronic ulcerative colitis (355). Some tumors are multicentric (355). The patients have colitis for 2 to 20 years (355).
Schistosomal ova are abundant throughout the bowel wall associated with fibrous proliferations (Fig. 14.4).
Schistosomal ova are abundant throughout the bowel wall associated with fibrous proliferations (Fig. 14.4).
Special Histologic Types of Colorectal Carcinoma
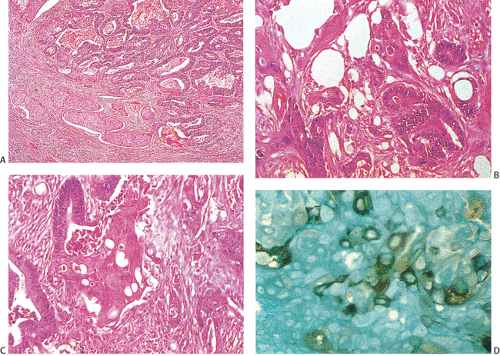
Mucinous Carcinomas
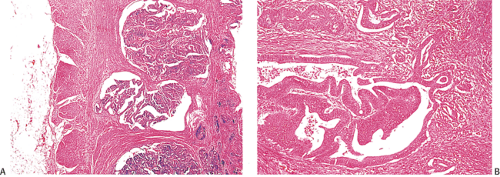
Many ordinary adenocarcinomas contain a mucinous component (Figs. 14.110 and 14.111), but when >50% of the tumor is mucinous, the tumor is classified as a mucinous adenocarcinoma (314,356,357). Mucinous tumors account for 10% to 15% of colorectal carcinomas (358,359) and 33% of rectal cancers (358). There are two subtypes of mucinous carcinoma: Colloid carcinoma and signet ring cell carcinoma. In colloid carcinomas, the mucin is extracellular, whereas in signet ring carcinomas, the mucin is intracellular in location. In both types, the material secreted is an acid mucopolysaccharide that stains with periodic acid–Schiff (PAS), mucicarmine, and acidic aniline blue stains. Like ordinary adenocarcinomas, both signet ring carcinomas (Fig. 14.112) and colloid carcinomas arise in adenomas, particularly villous adenomas (358,360). Residual adenoma may be present in up to 31% of mucinous carcinomas (358,360). Mucinous carcinomas sometimes metastasize within the intestinal mucosa, creating many new polypoid lesions in the remaining mucosa. One extraordinary example of this was a patient who had >100 “polyps” (361).
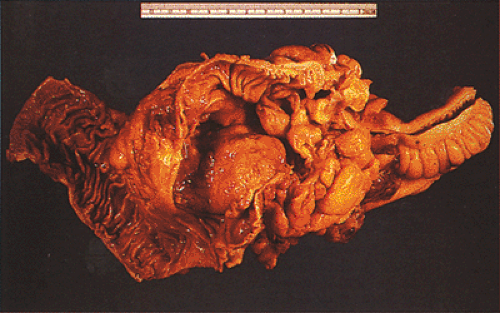 FIG. 14.110. The colon contains a large fungating mucinous tumor. Grossly, the mucinous areas may be appreciated on cut section, where they appear soft and gelatinous (not shown). |
Mucinous carcinomas differ clinically and pathologically from ordinary carcinomas. These tumors often affect young patients (314) and those with HNPCC. Seventy-nine to eighty-three percent of colorectal cancers in patients in the first 3 decades of life are predominantly mucinous, approximately equally divided between the signet ring and the colloid types (307,314). The delay in diagnosis in young people accounts for the very poor prognosis of mucinous carcinomas, especially in young patients (314,358). Mucinous carcinomas are more likely to invade adjacent viscera (29% vs. 10%) and show more extensive lymph node involvement beyond the pericolonic region (50% vs. 26%) than nonmucinous carcinomas (362). Disease recurrences occur more frequently in patients with colloid (51.7%) or signet ring carcinoma (100%) as compared
with ordinary adenocarcinomas. This justifies a more aggressive surgical approach, including extensive nodal dissection and resection of adjacent organs that seem to be macroscopically affected (362). Some patients with mucinous carcinomas develop pseudomyxoma peritonei or Trousseau syndrome (venous thrombophlebitis in patients with carcinoma). The latter may lead to cerebrovascular accidents (363).
with ordinary adenocarcinomas. This justifies a more aggressive surgical approach, including extensive nodal dissection and resection of adjacent organs that seem to be macroscopically affected (362). Some patients with mucinous carcinomas develop pseudomyxoma peritonei or Trousseau syndrome (venous thrombophlebitis in patients with carcinoma). The latter may lead to cerebrovascular accidents (363).
Overall, mucinous carcinomas have a significantly worse prognosis, with a 5-year survival rate of 17% to 18% and a median survival of 33 months (364). The prognosis is worst in rectal mucinous tumors (358,359,365,366). In one study, the prognosis was found to be worse with tumors of high mucin content (>80%) than in those with a moderate mucinous content (60% to 80%) (366). The poor prognosis results in part from the tendency of mucinous tumors to dissect through the bowel wall in an infiltrative pattern.
Finding a significant mucinous component in the preoperative biopsy correlates well with similar findings in the resection specimen. In one study, 83% of mucinous carcinoma–positive biopsy specimens exhibited >25% mucinous carcinoma in the corresponding resection specimen, whereas only 10% of biopsies negative for mucinous carcinoma contained >25% mucinous carcinoma in the surgical specimen. Similarly, 83% of mucinous carcinoma–positive biopsy specimens revealed carcinoma at the B2 or higher stage upon resection compared with 63% of mucinous carcinoma–negative biopsy specimens (356).
Colloid Carcinomas
Colloid carcinomas represent 10% to 15% of all carcinomas of the large bowel (358,359). Colloid carcinomas often arise in villous adenomas (367,379), in areas of villous dysplasia in ulcerative colitis (358), or in an anorectal fistula (368). The tumors may appear as very large, bulky, gelatinous neoplasms lacking fibrosis or scarring but containing large mucinous areas. The mucinous pools allow the tumor to dissect easily along tissue planes. The presence of the mucin makes surgical extirpation difficult (357).
Colloid carcinomas are diagnosable when one sees (a) malignant-appearing glands disrupted by the presence of abundant, extruded, intraluminal mucin; (b) mucin pools in the connective stroma; and (c) superficial pools of mucin containing free-lying ribbons or clusters of malignant cells. Columnar cells may line the large mucinous pools (Figs. 14.113 and 14.114). The epithelium may appear extremely well differentiated. Other tumors consist of irregular glands, trabecular groups of cells, or clumps of cells lying free in
mucinous lakes. Colloid carcinomas may demonstrate extensive lymphatic involvement or extensive submucosal spread, appearing to track along the submucosa and undermining the mucosa (369). Colloid carcinomas exhibit a propensity for lymph node metastases, reduced tubule differentiation, less lymphocytic infiltration, and more proximal distribution compared with nonmucinous tumors (367,370). A tendency for proximal distribution probably associates with the finding that HNPCC patients tend to have proximal lesions that exhibit a mucinous histology.
mucinous lakes. Colloid carcinomas may demonstrate extensive lymphatic involvement or extensive submucosal spread, appearing to track along the submucosa and undermining the mucosa (369). Colloid carcinomas exhibit a propensity for lymph node metastases, reduced tubule differentiation, less lymphocytic infiltration, and more proximal distribution compared with nonmucinous tumors (367,370). A tendency for proximal distribution probably associates with the finding that HNPCC patients tend to have proximal lesions that exhibit a mucinous histology.
Signet Ring Cell Carcinomas
The second type of mucinous tumor is the “intracellular” type or signet ring cell carcinoma. The signet ring variety of mucinous carcinoma, although uncommon, tends to affect younger individuals. Patients present with changes in bowel habits, weight loss, and blood and mucus in the stools. Signet ring cell carcinomas account for approximately 1.1% of all colorectal carcinomas (364). Patients range in age from 18 to 29 years (360). Patients with signet ring carcinoma tend to
have more extensive disease at the time of diagnosis than do those with ordinary adenocarcinoma (371). This has been attributed to delay in diagnosis, because many of the patients are young and the disease clinically resembles IBD. As many as 30% of patients with signet ring carcinoma have ulcerative colitis, further complicating recognition of the cancer (372). Furthermore, the tumor tends to spread intramurally with relative sparing of the mucosa, making it difficult to see radiographically, endoscopically, or grossly. There are few symptoms early in its course. The diffuse intramural infiltration by neoplastic cells may, at first, suggest an inflammatory process. Diffuse and circumferential bowel wall infiltration produces a thick, rigid, intestinal segment creating colonic linitis plastica (372). Advanced lesions present with constriction of the intestinal wall, widespread lymph node metastases, and spread to the peritoneal surfaces.
have more extensive disease at the time of diagnosis than do those with ordinary adenocarcinoma (371). This has been attributed to delay in diagnosis, because many of the patients are young and the disease clinically resembles IBD. As many as 30% of patients with signet ring carcinoma have ulcerative colitis, further complicating recognition of the cancer (372). Furthermore, the tumor tends to spread intramurally with relative sparing of the mucosa, making it difficult to see radiographically, endoscopically, or grossly. There are few symptoms early in its course. The diffuse intramural infiltration by neoplastic cells may, at first, suggest an inflammatory process. Diffuse and circumferential bowel wall infiltration produces a thick, rigid, intestinal segment creating colonic linitis plastica (372). Advanced lesions present with constriction of the intestinal wall, widespread lymph node metastases, and spread to the peritoneal surfaces.
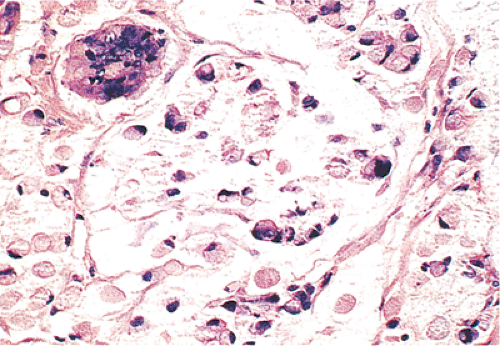
The histologic diagnosis of signet ring carcinoma depends on finding a preponderance (>50%) of poorly differentiated tumor cells with copious intracytoplasmic mucin pushing the nuclei to the cell periphery (Figs. 14.115 and 14.116). Some tumor cells may appear extremely anaplastic. However, not all signet ring cell carcinomas display a classic signet ring morphology. Rather, they contain cells with a fine, foamy cytoplasm and a central nucleus (Fig. 14.117).
The differential diagnosis of signet ring cell carcinomas involves a primary glandular neoplasm, a metastasis from another site such as the stomach or breast (Fig. 14.118), and adenocarcinoid tumors. Since linitis plastica with signet ring carcinoma has its greatest frequency in the stomach, secondary involvement of the colon by a gastric cancer must be considered when colonic linitis plastica is encountered. If residual adenoma is present, one can be reasonably confident that the lesion is primary, although we have seen rare cancers metastatic to an adenomatous polyp (373). Patient history and immunostains are extremely important in ruling out a metastatic tumor. Adenocarcinoid tumors tend to grow in a more organoid fashion than signet ring cell carcinomas, and the cells appear more cohesive than the widely separated, more discohesive cells typical of colonic signet ring cell tumors.
Overall, signet ring cell carcinomas behave aggressively (371,374), with most patients presenting at stage III or IV. The overall 5-year survival in one series was 9.4% (374). Seventy-five percent of patients with T2 disease survived 5 years, compared with 5.1% and 0% of patients with T3 and T4 tumors, respectively.
Linitis Plastica
The term linitis plastica (Greek for linen cloth or net) was first described by Lietaud in 1779, but credit for the first case is given to Andrau, who, in 1829, described the condition. Primary linitis plastica of the colon was first described by Laufman and Saphir (375). Since the term linitis plastica is a gross term, several different histologic forms of cancer may produce the changes.
Linitis plastica is characterized by diffuse infiltration of neoplastic cells into a hollow viscus with significant desmoplastic response, imparting a rigid, fibrotic, thickened appearance to the wall of the organ (375). Both primary and secondary carcinomas can cause a colonic linitis plastica. Some cases of linitis plastica occur secondary to involvement by a gastric carcinoma (376). Grossly, the intestine appears diffusely thickened, particularly in the area of the submucosa. Eighty percent of primary lesions develop distal to the splenic flexure. In contrast, linitis plastica resulting from a metastatic gastric carcinoma usually affects the transverse colon, since metastases occur hematogenously via the gastrocolic ligament (377).
Linitis plastica affects individuals from age 20 to 60 years, with an average age of 51 to 56 years (377,378). The clinical features are usually limited to lower gastrointestinal symptoms, most often including altered bowel habits and abdominal or rectal pain, or melena. The average symptom duration prior to diagnosis is 3 months (377). Some patients have pre-existing ulcerative colitis (377).
Radiologically, the disease simulates primary inflammatory disorders, such as Crohn disease, diverticulitis, and others. The mucosa may be relatively spared by the tumor, with the majority of the lesion infiltrating the submucosa (375). Radiologically, linitis plastica lacks an intraluminal mass or areas of ulceration. Rather, the bowel wall appears diffusely thickened. These changes may be seen more easily on computed tomography (CT) or magnetic resonance imaging (MRI) than on a barium enema.
Because there is frequently a lack of an apparent mucosal lesion, endoscopically directed biopsies should be deep enough to include the submucosa in order to provide a more accurate diagnosis. Grossly, the margins of the lesion are tapered and appear poorly delineated. The mucosa usually
appears intact, although it may be ulcerated. Histologically, linitis plastica consists of diffusely infiltrating, darkly stained, small, atypical cells that may have a signet ring appearance (Fig. 14.119). Abortive gland formation may also be present. The tumors spread early, via lymphatic dissemination and/or by local extension (Fig. 14.120). Both peritoneal seeding and regional lymph node metastases are often present at the time of initial surgery. Seventy-five percent of patients have lymph
nodal metastases, and 38% have peritoneal metastases. Females often have metastases to the uterus, ovaries, and fallopian tubes. Diffuse carcinomatosis of the abdomen or pelvis frequently occurs (378). Liver metastases are present in a low percentage of patients. Spread to bone and lung is rare (377). The prognosis is poor, with a mean duration of survival of only 8.3 months after diagnosis (377).
appears intact, although it may be ulcerated. Histologically, linitis plastica consists of diffusely infiltrating, darkly stained, small, atypical cells that may have a signet ring appearance (Fig. 14.119). Abortive gland formation may also be present. The tumors spread early, via lymphatic dissemination and/or by local extension (Fig. 14.120). Both peritoneal seeding and regional lymph node metastases are often present at the time of initial surgery. Seventy-five percent of patients have lymph
nodal metastases, and 38% have peritoneal metastases. Females often have metastases to the uterus, ovaries, and fallopian tubes. Diffuse carcinomatosis of the abdomen or pelvis frequently occurs (378). Liver metastases are present in a low percentage of patients. Spread to bone and lung is rare (377). The prognosis is poor, with a mean duration of survival of only 8.3 months after diagnosis (377).
Squamous Cell–containing Carcinomas
The incidence of squamous and adenosquamous carcinoma ranges between 0.1 and 0.5 cases per 1,000 colonic malignancies (379,380). The tumors show an increased incidence in patients with chronic ulcerative colitis (379). Squamous cell carcinomas may also develop in patients with Crohn disease and longstanding rectal fistulae. The tumors that arise in the setting of IBD may result from hyperstimulation of intestinal stem cells due to an ongoing chronic inflammatory response. This could lead to stem cell hyperplasia and a broad spectrum of tumor types, including those containing squamous differentiation. A simple proliferation of basal cells may replace the damaged epithelium. Further demands for cell replacement lead to basal cell anaplasia and the inability to differentiate normally, resulting in tumors that become glandular, become squamous, or show mixed cell populations. In this setting, the carcinoma need not develop in a pre-existing adenoma. Other adenosquamous and squamous carcinomas arise in adenomas, perhaps from those containing squamous morules (Fig. 14.36). Pure squamous cell carcinomas occur less commonly than adenoacanthomas or adenosquamous cancers.
Both sexes are equally affected (380). Hypercalcemia complicates the clinical picture in some patients due to production of parathormone by the tumor (381,382,383). Humoral calcemia of malignancy is diagnosed on the basis of an elevated parathormone level and positive tumor immunoreactivity to parathormone hormone (381). Colonic tumors containing areas of squamous differentiation are treated in the same fashion as ordinary adenocarcinomas.
Adenosquamous Carcinomas and Adenoacanthomas
No gross features distinguish adenosquamous carcinomas or adenoacanthomas from ordinary adenocarcinomas. Adenosquamous carcinomas usually occur in young patients who
tend to present with advanced disease. They follow a highly aggressive course when compared with classic colorectal adenocarcinomas (380,384), with an overall 5-year survival of approximately 30% (384). Distant metastases occur early.
tend to present with advanced disease. They follow a highly aggressive course when compared with classic colorectal adenocarcinomas (380,384), with an overall 5-year survival of approximately 30% (384). Distant metastases occur early.
Histologically, adenosquamous carcinomas consist of an admixture of malignant glands and malignant squamous cells with variable keratinization (Fig. 14.121). Rarely, the tumors combine a mucinous and/or signet ring cell adenocarcinoma with a squamous cell component (385). The tumors often show extensive lymphatic invasion. When the tumors metastasize, the metastases may contain both glandular and squamous cell components or either component by itself. Rare tumors combine adenosquamous elements with areas of carcinoid differentiation. In this situation, the three elements intimately intermingle with one another. Such lesions exhibit the biologic behavior of the underlying adenosquamous carcinoma and not the carcinoid tumor.
Adenoacanthomas refer to those adenocarcinomas that contain a benign squamous cell component. These lesions are not grossly distinctive. Adenoacanthomas account for <0.2% of all colonic malignancies (386).
Squamous Carcinomas
The following criteria are required to diagnose a primary intestinal squamous cell carcinoma (387): (a) no evidence of primary squamous carcinoma exists in any other site that could provide a source of metastasis or direct extension to the bowel, (b) the affected bowel segment is not a squamous-lined fistula, (c) no continuity exists between the tumor and the anal squamous epithelium, and (d) no glandular differentiation is present. When these strict diagnostic criteria are met, squamous carcinomas of the colorectum are exceedingly rare. A recent estimate from SEER data in United States demonstrated that squamous cell carcinoma comprised 0.3% of all colorectal carcinomas (388). Squamous cell carcinomas develop in patients who have had previous radiation therapy (389), ulcerative colitis (389,390,391), schistosomiasis (389,392), congenital malformations (393), and chronic sinuses. These associations suggest that chronic inflammation stimulates reserve cells in the crypt to become squamous and neoplastic. Some patients have metachronous colonic carcinomas (394).
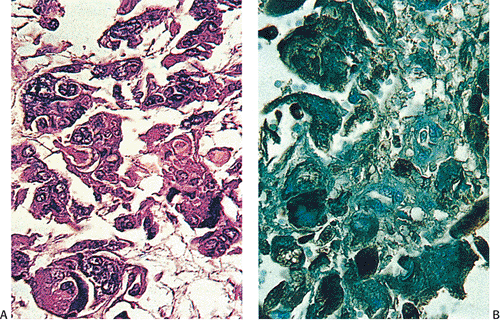 FIG. 14.122. Colon cancer. A: Carcinoma with choriocarcinomatous differentiation. B is stained with antibody to human chorionic gonadotropin. |
No gross features identify a colon cancer as a squamous carcinoma. Most squamous carcinomas are large, ulcerated lesions. They may be confined to a small polyp (395). Most tumors arise in the rectum, but they may also arise in other colonic sites (388). The tumors range in differentiation from well to poorly differentiated. Histologically, well-differentiated intestinal squamous cell carcinomas show prominent areas of keratinization, intercellular bridges, keratin pearls, lack of gland formation, and no stainable mucin. Keratin formation may be inconspicuous in poorly differentiated tumors. The tumors metastasize to the regional lymph nodes and to the liver (389). The overall 5-year survival for colorectal squamous cell carcinomas is 49% (388).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree