CHAPTER 10 Acute Abdominal Pain
Acute abdominal pain is a common complaint that brings patients to emergency departments. As many as 1 in 20 emergency department visits is for abdominal pain.1 Approximately half of these patients have nonspecific findings or “gastroenteritis.”2 The other half have a more serious disorder that warrants further evaluation and treatment. A small proportion of patients has a life-threatening disease. Therefore, the evaluation of acute abdominal pain must be efficient and lead to an accurate diagnosis early in the presentation so that the treatment of patients who are seriously ill is not delayed and patients with self-limited disorders are not overtreated. This chapter discusses the anatomic factors that determine how abdominal pain is perceived, a systematic approach to the evaluation of abdominal pain, and common and special circumstances encountered in evaluating patients with acute abdominal pain.
ANATOMY
Physiologic determinants of pain include the nature of the stimulus, the type of receptor involved, the organization of the neural pathways from the site of injury to the central nervous system, and a complex interaction of modifying influences on the transmission, interpretation, and reaction to pain messages.3,4 Sensory neuroreceptors in abdominal organs are located in the mucosa and muscularis of hollow viscera, on serosal structures such as the peritoneum, and within the mesentery.5 In addition to nociception (the perception of noxious stimuli), sensory neuroreceptors are involved in the regulation of secretion, motility, and blood flow via local and central reflex arcs.6 Although sensory information conveyed in this manner usually is not perceived, disordered regulation of these gastrointestinal functions (secretion, motility, blood flow) can cause pain. For example, patients with irritable bowel syndrome perceive pain as a result of heightened sensitivity of intestinal afferent neurons to normal endogenous stimuli that results in altered gut motility and secretion (see Chapter 118).7
VISCERAL PAIN
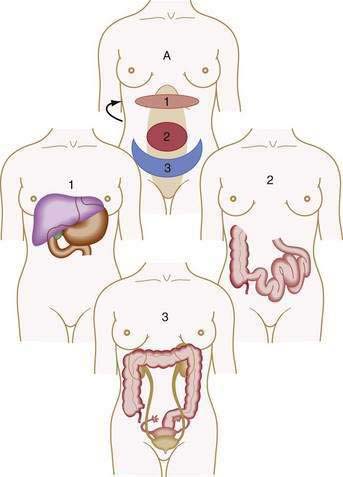
Visceral pain is transmitted by C fibers that are found in muscle, periosteum, mesentery, peritoneum, and viscera. Most painful stimuli from abdominal viscera are conveyed by this type of fiber and tend to be dull, cramping, burning, poorly localized, and more gradual in onset and longer in duration than somatic pain. Because abdominal organs transmit sensory afferents to both sides of the spinal cord, visceral pain is usually perceived to be in the midline, in the epigastrium, periumbilical region, or hypogastrium (Fig. 10-1). Visceral pain is not well localized because the number of nerve endings in viscera is lower than that in highly sensitive organs such as the skin and because the innervation of most viscera is multisegmental. The pain is generally described as cramping, burning, or gnawing. Secondary autonomic effects such as sweating, restlessness, nausea, vomiting, perspiration, and pallor often accompany visceral pain. The patient may move about in an effort to relieve the discomfort.
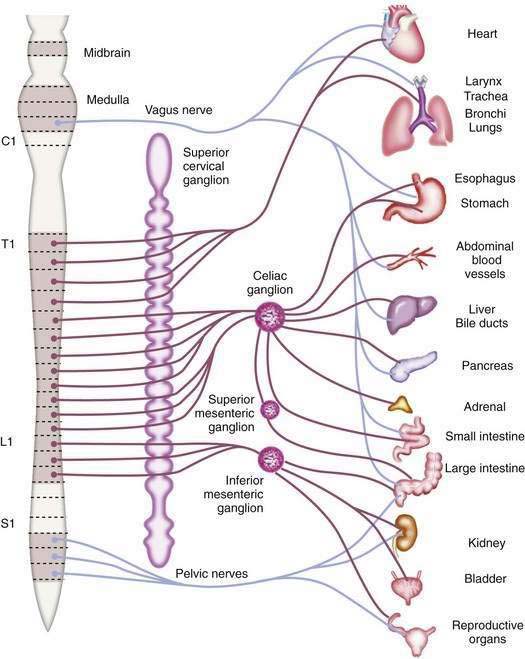
The afferent fibers that mediate painful stimuli from the abdominal viscera follow the distribution of the autonomic nervous system (Fig. 10-2). The cell bodies for these fibers are located in the dorsal root ganglia of spinal afferent nerves. On entering the spinal cord, these fibers branch into the dorsal horn and tract of Lissauer, where afferent nerves from adjacent spinal segments travel in a cephalad direction and caudally over one or two spinal segments before terminating on dorsal horn cells in laminae I and V. The dorsal horn cells within laminae I and V are the primary projection neurons for ascending pain pathways. From the dorsal horn, second-order neurons transmit nociceptive impulses via fibers that pass across the anterior commissure and ascend the spinal cord in the contralateral spinothalamic tract. These fibers project to the thalamic nuclei and the reticular formation nuclei of the pons and medulla. The thalamic nucleus sends third-order neurons to the somatosensory cortex, where the discriminative aspects of pain are perceived. The reticular formation nucleus sends neurons to the limbic system and frontal cortex, where the emotional aspects of pain are interpreted.8,9
Abdominal visceral nociceptors respond to mechanical and chemical stimuli. The principal mechanical signal to which visceral nociceptors are sensitive is stretch; cutting, tearing, or crushing of viscera does not result in pain. Visceral stretch receptors are located in the muscular layers of the hollow viscera, between the muscularis mucosa and submucosa, in the serosa of solid organs, and in the mesentery (especially adjacent to large vessels).5,10 Mechanoreceptor stimulation can result from rapid distention of a hollow viscus (e.g., intestinal obstruction), forceful muscular contractions (e.g., biliary pain or renal colic), and rapid stretching of solid organ serosa or capsule (e.g., hepatic congestion). Similarly, torsion of the mesentery (e.g., cecal volvulus) or tension from traction on the mesentery or mesenteric vessels (e.g., retroperitoneal or pancreatic tumor) results in stimulation of mesenteric stretch receptors.
Abdominal visceral nociceptors also respond to various chemical stimuli. Chemical nociceptors are contained mainly in the mucosa and submucosa of the hollow viscera. These receptors are activated directly by substances released in response to local mechanical injury, inflammation, tissue ischemia and necrosis, and noxious thermal or radiation injury. Such substances include H+ and K+ ions, histamine, serotonin, bradykinin and other vasoactive amines, substance P, calcitonin gene-related peptide, prostaglandins, and leukotrienes.11,12 Accumulation of nociceptor-reactive substances may change the microenvironment of the injured tissue and thereby reduce the pain threshold. The sensation of pain to a given stimulus is thus increased, and otherwise innocuous stimuli become painful. For example, the application of chemical irritants or pressure to normal gastric mucosa is not painful, whereas the application of the same stimuli to inflamed or injured gastric mucosa causes pain.
SOMATIC-PARIETAL PAIN
Somatic-parietal pain is mediated by A-δ fibers that are distributed principally to skin and muscle. Signals from this neural pathway are perceived as sharp, sudden, well-localized pain, such as that which follows an acute injury. These fibers convey pain sensations through spinal nerves. Stimulation of these fibers activates local regulatory reflexes mediated by the enteric nervous system and long spinal reflexes mediated by the autonomic nervous system, in addition to transmitting pain sensation to the central nervous system.13
Reflexive responses, such as involuntary guarding and abdominal rigidity, are mediated by spinal reflex arcs involving somatic-parietal pain pathways. Afferent pain impulses are modified by inhibitory mechanisms at the level of the spinal cord. Somatic A-δ fibers mediate touch, vibration, and proprioception in a dermatomal distribution that matches the visceral innervation of the injured viscera and synapse with inhibitory interneurons of the substantia gelatinosa in the spinal cord. In addition, inhibitory neurons that originate in the mesencephalon, periventricular gray matter, and caudate nucleus descend within the spinal cord to modulate afferent pain pathways. These inhibitory mechanisms allow cerebral influences to modify afferent pain impulses.9,14
REFERRED PAIN
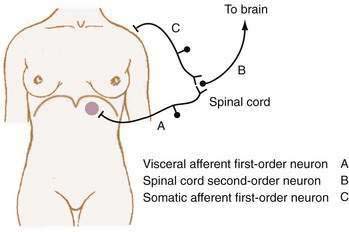
Referred pain is felt in areas remote from the diseased organ and results when visceral afferent neurons and somatic afferent neurons from a different anatomic region converge on second-order neurons in the spinal cord at the same spinal segment. This convergence may result from the innervation, early in embryologic development, of adjacent structures that subsequently migrate away from each other. As such, referred pain can be understood to refer to an earlier developmental state. For example, the central tendon of the diaphragm begins its development in the neck and moves craniocaudad, bringing its innervation, the phrenic nerve, with it.15 Figure 10-3 shows how diaphragmatic irritation from a subphrenic hematoma or splenic rupture may be perceived as shoulder pain (Kehr’s sign).9
CLINICAL EVALUATION
APPROACH TO ACUTE CARE
HISTORY
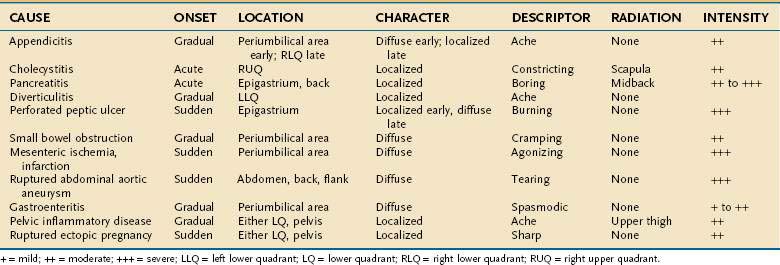
Despite the advances made in clinical imaging, history taking remains the most important component of the initial evaluation of the patient with acute abdominal pain.16 Characteristic features of the pain associated with various common causes of acute abdominal pain are shown in Table 10-1. Attention to these features can lead to a rapid clinical diagnosis or exclusion of important diseases in the differential diagnosis, thus enhancing the reliability and effectiveness of subsequent diagnostic testing.2
Chronology
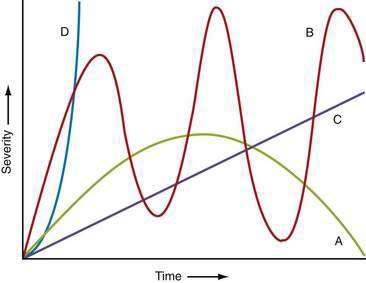
The time courses of several common causes of acute abdominal pain are diagrammed in Figure 10-4. The rapidity of onset of pain is often a measure of the severity of the underlying disorder. Pain that is sudden in onset, severe, and well localized is likely to be the result of an intra-abdominal catastrophe such as a perforated viscus, mesenteric infarction, or ruptured aneurysm. Affected patients usually recall the exact moment of onset of their pain. Progression is an important temporal factor in abdominal pain. In some disorders, such as gastroenteritis, pain is self-limited, whereas in others, such as appendicitis, pain is progressive. Colicky pain has a crescendo-decrescendo pattern that may be diagnostic, as in renal colic. The duration of abdominal pain is also important. Patients who seek evaluation of abdominal pain that has been present for an extended period (e.g., weeks) are less likely to have an acute life-threatening illness than patients who present within hours to days of the onset of their symptoms.
Location
The location of abdominal pain provides a clue to interpreting the cause. As noted, a given noxious stimulus may result in a combination of visceral, somatic-parietal, and referred pain, thereby creating confusion in interpretation unless the neuroanatomic pathways are considered. For example, the pain of diaphragmatic irritation from a left-sided subphrenic abscess may be referred to the shoulder and misinterpreted as pain from ischemic heart disease (see Fig. 10-3). Changes in location may represent progression from visceral to parietal irritation, as with appendicitis, or represent the development of diffuse peritoneal irritation, as with a perforated ulcer.