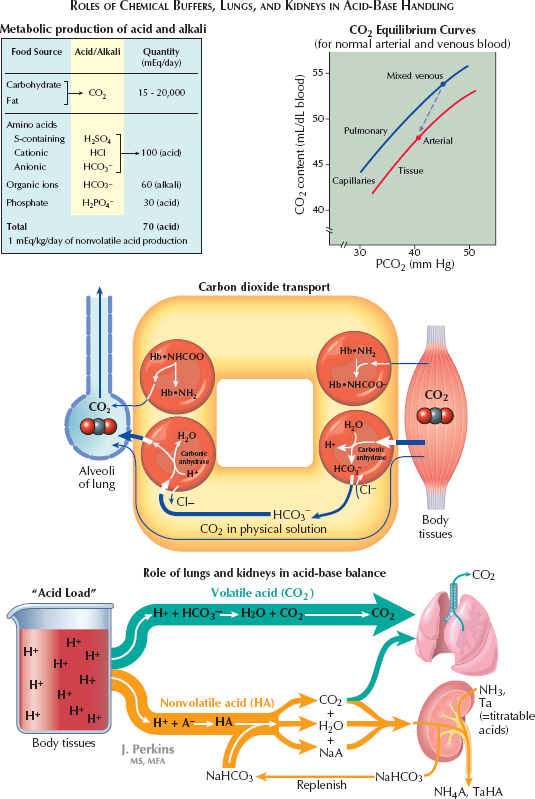
In this manner, the constant generation of carbon dioxide could lead to a significant acid load. Because carbon dioxide is a volatile gas, however, it can be excreted from the lung, which minimizes its impact. From the peripheral tissues, CO2 can reach the lung in multiple different manners. First, it may simply be dissolved in plasma. Second, it can enter erythrocytes and become bound to hemoglobin. Finally, and most importantly, it can enter erythrocytes and be converted by carbonic anhydrase into a proton, which is buffered within the cell by hemoglobin, and bicarbonate, which is secreted into the plasma in exchange for chloride.
The metabolism of proteins, in contrast, generates nonvolatile sulfur- and phosphate-based acids, which cannot be directly excreted from the lungs. To neutralize such acids, the extracellular fluid contains buffers that can bind or release protons as needed, so as to minimize fuctuations in the overall proton concentration. The most important extracellular buffer is bicarbonate (HCO3−), which can receive a free proton and be converted back into water and carbon dioxide, which is then excreted from the lungs. The ventilation rate is centrally modulated in response to fuctuations in arterial pH so that as more protons are added to the blood, more carbon dioxide is eliminated from the lungs.
The effect of this system can be demonstrated as follows. Because carbonic acid is unstable, the above equation can be simplified as follows:
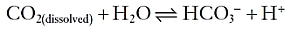
The equilibrium constant, Ka, is equal to:
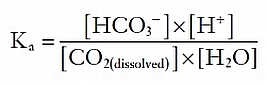
If Ka’ is defined as Ka * [H2O], then additional rearrangement gives:
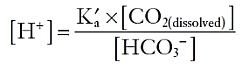
< div class='tao-gold-member'>




