Titratable Acids. Urine contains several weak acids that serve as urinary buffers. These buffers are also called “titratable acids” because their concentration can be determined based on how much NaOH is required to titrate collected urine to a pH of 7.4.
Phosphate, which exists in plasma primarily as HPO42-, is the major titratable acid in urine because its pKa of 6.8 is near physiologic pH. Using the Henderson-Hasselbalch equation, the ratio of protonated to unprotonated species can be expressed as follows:
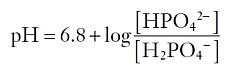
At a pH of 7.4, the ratio of HPO42- to H2PO4 is 4:1 based on the above equation. Thus, four fifths of the filtered phosphate is available to buffer protons.
Most of the H2PO4 is protonated in the collecting duct. As described previously, type A intercalated cells possess cytoplasmic carbonic anhydrase II, which converts carbon dioxide and water to bicarbonate ions, which are reabsorbed, and protons, which are secreted. Some of the secreted protons contribute to bicarbonate reabsorption, as described previously, but most combine with buffers such as HPO42~. The overall process leads to net synthesis of bicarbonate ions and excretion of protons.
< div class='tao-gold-member'>




