At normal body temperature, the concentration of dissolved carbon dioxide is equal to 3% of its partial pressure. By substitution, and slight further rearrangement:
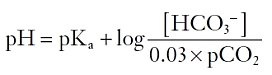
This formula, an expression of the general Henderson-Hasselbalch equation, demonstrates that as bicarbonate is depleted and carbon dioxide generated, changes in pH are minimized if the partial pressure of carbon dioxide is kept constant or even lowered. Thus, in normal circumstances, the respiratory rate is adjusted so that PCO2 remains constant at about 40 mm Hg.
For this system to remain sustainable, however, a new supply of free bicarbonate ions must be created to replenish the depleted extracellular buffers. Such an event occurs in the renal tubules, where free bicarbonate ions are generated and their paired protons excreted.
KIDNEYS
The kidneys are responsible both for reabsorbing existing bicarbonate ions and for generating new bicarbonate ions. The latter process is, by necessity, paired with the excretion of protons, since otherwise the newly generated bicarbonate would simply be converted back into carbon dioxide.
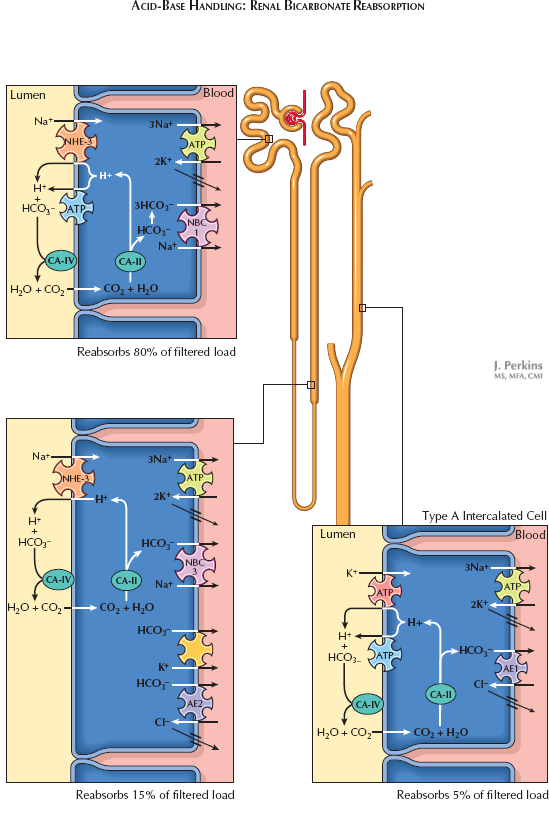
Bicarbonate Reabsorption. Bicarbonate is freely filtered at the glomerulus. About 80% of the filtered load is reabsorbed in the proximal tubule. The process begins in the cytoplasm of the tubular epithelium, where carbonic anhydrase II catalyzes the conversion of carbon dioxide and water into bicarbonate ions and protons. The bicarbonate ions are reabsorbed into the interstitium on basolateral NBC-1 Na+/HCO3− cotransporters; the protons, meanwhile, are secreted across the apical surface on NHE-3 Na+/H+ exchangers and, to a lesser extent, H+ ATPases. Once the protons are in the tubular fluid, membrane-bound carbonic anhydrase IV catalyzes their reaction with filtered bicarbonate ions to produce carbon dioxide and water. The newly formed carbon dioxide diffuses into proximal tubule cells, and the entire process begins again. Note that there is no net proton secretion during this process because protons are recycled across the apical membrane to capture filtered bicarbonate.
About 15% of the filtered bicarbonate load is reabsorbed in the thick ascending limb. The process in this segment is comparable to that seen in the proximal tubule; however, there does not appear to be a significant role for apical H+ pumps, the basolateral NBC transporter is of a different isoform, and the basolateral membrane also features Cl −/HCO3– exchangers (AE2) and K+/HCO3− symporters.
Nearly all of the remaining bicarbonate is reabsorbed in the collecting duct. Type A intercalated cells are responsible for this process. Like the cells in earlier segments, they possess cytoplasmic carbonic anhydrase II, which converts carbon dioxide and water to bicarbonate ions and protons. The protons, however, are secreted in an Na-independent manner using H+ ATPases and H+/K+ ATPases, while the bicarbonate is reabsorbed on Cl−/HCO3− exchangers (AE1, also known as band 3 protein).
< div class='tao-gold-member'>




