Fig. 26.1
Patient candidate for abdominal wall transplantation. The wall is severely damaged from repeated laparotomies and enterocutaneous fistulae
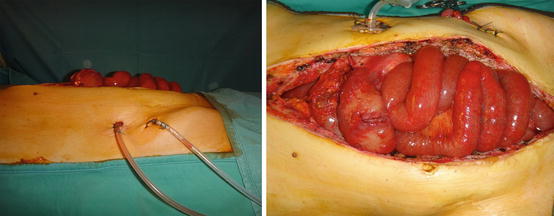
Fig. 26.2
At the end of bowel transplantation, the recipient abdominal wall is not able to properly cover the graft
The main problems that may lead to a difficult abdominal closure are [2, 3]:
Multiple previous laparotomies
Enterocutaneous fistulae
Donor to recipient unfavourable weight ratio
Postoperative edema
Presence of scar and fibrosis from previous surgery
Furthermore, in this kind of transplantation, diversion stomata and intraperitoneal drains are required, leading to a challenging abdominal wall closure.
Abdominal closure under tension might result in a wide range of complications, such as wound dehiscence, infections, necrosis, exposure of bowel loops, vascular thrombosis of the graft, abdominal compartment syndrome, and respiratory complications [4].
For all these reasons, abdominal wall transplantation was proposed for closure of patients undergoing both intestinal and multivisceral transplantation. The use of a composite tissue allograft in such patients has the main advantage of solving a big problem without requiring further immunosuppression. Abdominal wall transplantation is a feasible and safe procedure: it allows primary closure of the abdomen, avoids the potential morbidity of exposed viscera, and permits early mobilization and rehabilitation of these patients. The technique was first described by Levi et al. in 2003 [5], and, nowadays, whenever possible, it is the first choice to overcome a difficult closure.
The procurement of the abdominal wall graft does not interfere with the procurement of other organs and tissues. Transplantation of the abdominal wall composite graft can take place during the intestinal transplant procedure or several days later with a graft from a different donor. Delaying implantation of the abdominal wall graft allows perioperative edema to diminish before abdominal closure and the patient’s condition to stabilize. This strategy may be preferable when the recipient has a particularly large defect in the abdominal wall: in these cases a temporary negative pressure therapy is applied to reduce the infection risk.
In 2006 [6], our group described the first cases of abdominal wall transplantation performed with microsurgical technique, comparing our approach and Levi’s one. Nowadays, the microsurgical approach is well defined, and the technique is standardized.
26.2 Surgical Technique
The abdominal wall composite graft described by Levi is a full-thickness, vascularized, myocutaneous free flap. In the original description, it consists of one or both rectus abdominis muscles, with the investing fascia, the overlying subcutaneous tissue, and the skin, and the blood supply is derived from the donor inferior epigastric vessels, left in continuity with the larger iliac vessels. Procurement of the graft was done as part of the cadaveric, heart-beating donor, multiorgan procurement procedure [7, 8]. The procedure began with a bisubcostal incision. Longitudinal incisions were made following both lateral edges of the rectus muscles. These incisions were continued into the groins bilaterally. The common iliac vessels were identified. Finally, a transverse, suprapubic incision was made, connecting the two longitudinal incisions. The abdominal wall graft was packed with ice in situ during other organs procurement; then the distal aorta was cannulated, and the graft was flushed with cold preservation solution. The graft was removed in one piece with the iliac vessels, with a short segment of distal aorta and inferior vena cava. Closure of the donor’s abdomen was facilitated by mobilizing skin and subcutaneous tissue flaps from the lateral abdomen and flanks.
The abdominal wall graft is transplanted as a separate organ. The inclusion of an abdominal wall graft added about 2 h to the procedure’s operative time. The vessels of the abdominal wall graft were implanted into the recipient’s common iliac artery and vein. Alternatively, the infrarenal aorta and inferior vena cava can be used as recipient vessels.
The graft was sutured in layers to the recipient’s abdominal wall during closure of the abdomen. The graft, with its long vascular pedicle, was rotated and positioned according to location of the abdominal wall defect. The skin of the abdominal wall graft was left intact; normal skin color indicated adequate perfusion. The flow through the inferior epigastric vessels of the graft was monitored with a handheld Doppler ultrasound device. Biopsies of the skin of the graft were undertaken randomly and when rejection was suspected on clinical grounds.
26.3 Microsurgical Technique
The microsurgical approach was introduced in S.Orsola-Malpighi Hospital in Bologna in 2006 [6]. Abdominal wall harvest is part of a multiorgan procurement. The flap consists in a median oval cutaneous isle extended from xiphoid to pubis and from one oblique muscle to the other; the flap is composed of cutaneous and subcutaneous tissues, both rectus abdominis muscles and a small part of the oblique ones, the deep muscular sheet, and parietal peritoneum (Fig. 26.3). The vascular pedicle consisted in the deep inferior epigastric arteries and veins, isolated bilaterally, if possible. The flap is designed and harvested by a microsurgeon. Flap harvesting starts with a superior incision made until trough skin and subcutaneous tissues including the deep fascia under rectus muscles; then the flap is dissected preserving laterally a small part of oblique muscles. The dissection is stopped at the inferior edge of the flap, and the abdominal flap is turned over to face downwards to allow the procurement of the other abdominal organs. During this phase, the flap is packed with cold water and ice, while the other organs are flushed with preservation solution during their harvesting. After that, the pedicles of the abdominal wall flap are sectioned at the origin from iliac vessels (Fig. 26.4). A further cold perfusion is performed through incannulation of the two epigastric arteries; the abdominal graft is then stored in a conservation container with ice. Donor site is repaired by direct closure, after generous undermining of the residual lateral abdomen and flanks tissues.
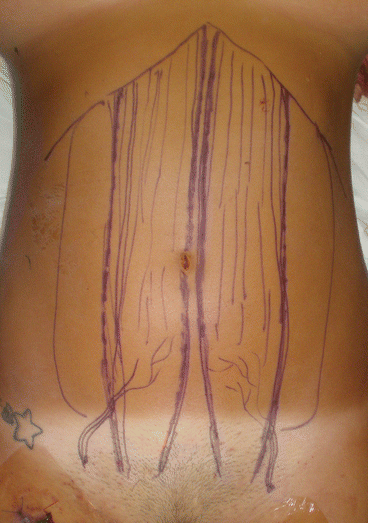
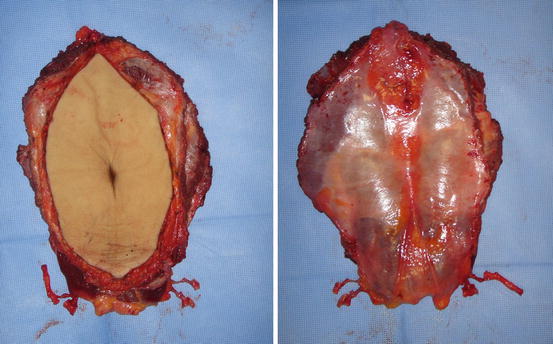

Fig. 26.3
Preoperative drawing on donor abdominal wall

Fig. 26.4
Abdominal wall flap at the end of harvesting from donor
The abdominal wall transplantation is performed after abdominal organs transplantation. The donor epigastric pedicles are anastomosed end-to-end with the recipient epigastric vessels or with the circumflex deep inferior vessels, as second choice (Fig. 26.5). Microsurgical abdominal wall transplantation procedure added about 2 h to the operative time. The flap is then sutured in multiple layers to the recipient residual abdominal wall. First the deep fascia layer is sutured, and then the oblique muscles, subcutaneous tissues, and skin are sutured. The patient is dressed leaving a window to allow continuous monitoring of flap vitality. Monitoring consists in a clinical follow-up considering capillary reflow and flap temperature. Skin biopsies are performed in the navel to monitor graft rejection every week for at least 1 month. Sutures are removed after 15 days (Fig. 26.6).
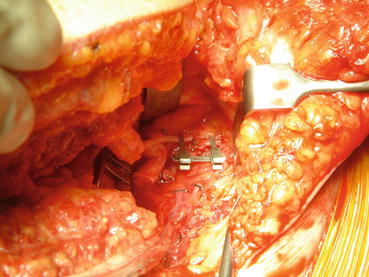
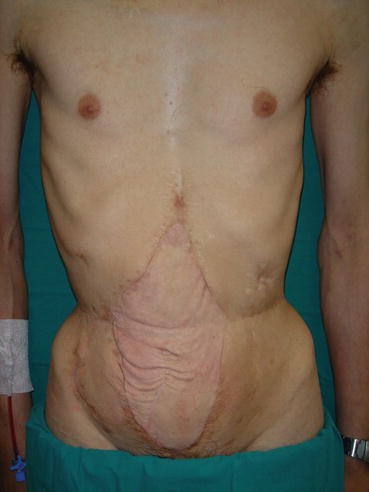

Fig. 26.5
End-to-end anastomosis between the graft pedicle and recipient epigastric inferior vessels

Fig. 26.6
Result after 4 years from abdominal wall transplantation
26.4 Discussion
Primary abdominal closure after intestinal or multivisceral transplantation is often impossible: the candidates to these transplantations have undergone multiple intestinal resections and present therefore a heavily scarred abdominal wall; moreover the abdominal cavity is often a virtual space [1, 9].
Another factor precluding a primary abdominal closure may be donor–recipient size mismatch. Fishbein et al. [10] reported that the most acceptable donor–recipient weight ratio is between 1.1 and 0.76, meaning that, ideally, donor and recipient sizes should be kept as close as possible. Several previous laparotomies and of partial/total enterectomy were predictive of difficult closure of the abdomen, even if the reported ratio is respected.
On the other hand, any attempt to close under tension might result in a wide range of complications, such as wound dehiscence, infections, necrosis of bowel loops, vascular thrombosis of the graft, abdominal compartment syndrome, and respiratory complications [4].
In such cases abdominal wall closure can be achieved with several methods: methods to reduce the graft volume, use of prosthetic mesh, previous abdominal wall expansion, pedicled flap, negative pressure therapy and subsequent skin graft, free flap, and abdominal wall transplantation.
There are several intraoperative maneuvers that can be performed routinely to reduce the graft volume and help achieve primary fascial closure.
Successful abdominal wall closure is most often obtained using donor that are 50–100 % the size of the recipient according to body weight. Overresuscitation with fluids should be avoided, and colloid solutions should be used if possible. Moreover all dysfunctional remnant bowels should be removed, and all adhesions should be lysed to completely develop all potential intraperitoneal space. Other space-creating options include splenectomy. However, caution is warranted because inferior outcomes were reported in recipients undergoing splenectomy mainly attributable to death from posttransplant sepsis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






