Vascular Abdominal Emergencies
Keywords
• Abdominal aorta • Mesenteric ischemia • Abdominal aortic aneurysm • Embolus • Arterial thrombus • Venous thrombus
Vascular abdominal anatomy
The aorta gives rise to several paired and unpaired vessels within the abdomen. The adrenal, renal, and gonadal arteries are paired, and provide blood flow to their respective organs. The unpaired branches (celiac artery, superior mesenteric artery [SMA], and inferior mesenteric artery [IMA]) deliver blood to most of the digestive tract. The celiac trunk branches off the aorta at approximately 90 degrees, making it less susceptible to embolic phenomena compared with the SMA and IMA. The 3 branches of the celiac trunk (the splenic, left gastric, and common hepatic arteries) supply the foregut structures from the distal esophagus to the second part of the duodenum, the spleen, the liver, and parts of the pancreas (Fig. 1).1
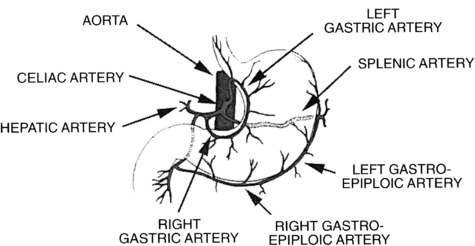
Fig. 1 The celiac artery and its 3 major branches: the splenic, left gastric, and hepatic arteries.
(From Walker JS, Dire DJ. Vascular abdominal emergencies. Emerg Med Clin North Am 1996;14(3):573; with permission.)
The SMA typically arises about 1 cm below the celiac trunk, at the approximate level of the first lumbar vertebra.2 The SMA gives rise to several branches that supply the midgut structures extending from the second part of the duodenum to the distal third of the transverse colon. The SMA leaves the aorta at an angle of less than 30 degrees, making it susceptible to thromboembolism (Fig. 2).1
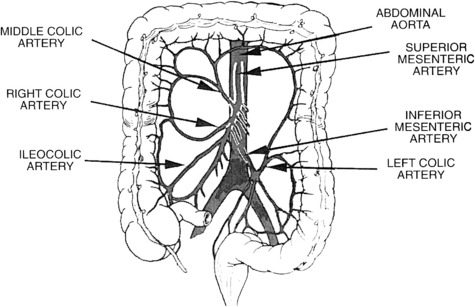
Fig. 2 The superior and inferior mesenteric arteries and their anastomotic connections.
(From Walker JS, Dire DJ. Vascular abdominal emergencies. Emerg Med Clin North Am 1996;14(3):574; with permission.)
Just before the bifurcation of the aorta at the level of the fourth lumbar vertebra, the IMA branches off the aorta and supplies all of the structures of the hindgut, which extend from the transverse colon to the rectum. It terminates in the superior rectal artery (see Fig. 2).2 Although each branch is separate, there is extensive collateral blood flow between the celiac artery and SMA via the pancreaticoduodenal artery, and between the SMA and the IMA by way of the marginal artery of Drummond and arc of Riolan.1
The venous system of the gastrointestinal tract differs from the arterial system in that, rather than draining into the inferior vena cava (IVC), it passes via the portal vein into the liver. Blood passes through the hepatic lobules into the hepatic veins, which pass into the IVC. In normal health, portal blood is sequestered from the systemic venous circulation. Pathologic conditions (eg, cirrhosis, obstruction, thrombosis) that obstruct portal flow cause anastomoses to form between systemic and portal vessels in tissues that are at the watershed junction between the 2 systems. These locations are the lower esophagus, the umbilicus, and the rectum.3 When anastomoses become very large they are referred to as varices.
Abdominal vascular thromboses
Aortic Thrombosis
Acute occlusive aortic thrombosis is a rare condition that is lethal if not diagnosed. Nonocclusive aortic thrombosis is more common and occurs in the setting of aneurysmal disease, dissection, or severe atherosclerotic disease.4–6 Other conditions associated with aortic thrombotic disease include diabetes, cardiomyopathy, blunt and penetrating abdominal trauma,7–9 spinal surgery, polycythemia,4 thrombocytosis,4 nephrotic syndrome, exogenous estrogens, the classic hypercoagulable conditions (protein C and S deficiency, factor V Leiden deficiency, antithrombin III deficiency), malignancy,5 use of certain chemotherapeutic agents,10 antiphospholipid antibody syndrome,5 and previous aortic grafting.4 Because of the large diameter of the aorta, emboli (typically from the left ventricle) rarely lead to aortic occlusion. Patients with an acute thrombotic event typically present with symptoms of lower extremity ischemia: bilateral lower extremity pulselessness, pallor, pain, paresthesias, and possible paralysis.4,11 Mesenteric ischemia may also be present. If the occlusion involves the artery of Adamkowicz, patients may also develop spinal cord infarction.12 Signs of the anterior spinal artery syndrome include paralysis and loss of sensation to both light touch and pinprick, but preservation of vibratory sensation and proprioception.12 In cases of chronic occlusion caused by atherosclerotic disease, there is time for the formation of collateral circulation to the distal structures.6 Leriche syndrome results from chronic obstruction of the distal aorta leading to chronic ischemia of the pelvis and lower extremities. Classically described in men, the triad of symptoms includes claudication, abnormal or absent lower extremity pulses, and erectile dysfunction.13,14 Acute ischemia from aortic occlusive disease warrants emergent consultation with a vascular surgeon. Patients will likely need emergent laparotomy for thrombectomy or embolectomy, often with aortic bypass.4 The cause of the thrombus and other associated conditions (eg, aneurysm or dissection) determine the vascular surgery approach to treatment. In one series of 14 patients, mortality after surgery was 14%.4 Overall mortality in a retrospective case series of 48 patients by Babu and colleagues15 was 52%. Dossa and colleagues11 reported a 40-year experience of 46 patients with an in-hospital mortality of 35%. The urgency of surgical consultation and intervention in patients with subacute or chronic occlusive disease is determined by the severity and progression of symptoms. Most patients will be admitted for observation, often with anticoagulant or antiplatelet agents.
Renal Artery Thrombosis
Renal artery thrombosis is also a rare condition, most commonly seen in individuals aged 30 to 50 years.16 Similar to the other thromboses, the most common cause involves the development of a clot in situ on established atherosclerotic lesions. Thrombosis is also a known complication after renal transplantation because of both the surgical anastomoses, and immunosuppressive drugs being prothrombotic.17 Intra-aortic catheter or balloon pump placement,18 intravenous (IV) cocaine use,16 and renal angiography are also risk factors. The transplanted renal artery graft may also develop thrombosis secondary to surgical technique including torsion of the artery, kinking of the anastomosis, or dissection into the wall.19 Large case series have described rates of thrombosis after transplant between 0.5% and 3.5%.19,20
Patients with acute renal artery occlusion typically present with flank pain, which may be associated with hypertension.16,21 Accompanying symptoms may include nausea, vomiting, and upper abdominal pain on the affected side. Hematuria only occurs in between 30% and 50% of patients.16 An untreated occlusion of the renal artery leads to renal infarction. In the setting of renal infarct, the serum lactate dehydrogenase (LDH) is typically increased.22 Symptoms may be difficult to distinguish from renal colic. When renal colic is suspected, unenhanced computed tomography (CT) is the initial study. However, with severe symptoms and a CT without renal stones, the study can be repeated with intravenous contrast if renal artery thrombosis is a consideration. In the renal transplant recipient, duplex ultrasound is the preferred initial test.
Renal artery occlusion can also occur in the setting of trauma. Blunt trauma to the abdomen can lead to compression of the artery, as well as dissection and thrombosis.23 Patients typically require surgical revascularization, and the timeliness of this intervention is linked with improved outcomes.21,24,25
Renal Vein Thrombosis
Renal vein thrombosis may be either an acute or chronic process. Diagnosis depends on a high level of clinical suspicion, because the clinical findings mimic those of renal colic, renal artery occlusion, and pyelonephritis.26 With acute thrombosis, patients experience flank pain often associated with nausea and vomiting. Hematuria and proteinuria may also be noted. In the setting of chronic thrombosis, the diagnosis may not be made until the development of complications such as impaired renal function or pulmonary embolism.27 The left renal vein is affected more often than the right, but up to two-thirds of patients have bilateral thrombosis.27
In contrast to occlusive arterial disease, renal vein thrombosis is also a disease of children and neonates. In the setting of severe volume depletion, dehydration, or sustained hypotension, blood flow is shunted from the renal vein, leading to sluggish flow that may eventually lead to the formation of a clot.26–28 In children, a palpable mass in the flank may be present because of the enlargement of the kidney on the affected side.28 The classic triad of symptoms in children includes a palpable mass, gross hematuria, and thrombocytopenia, although most patients do not have all three.29
Thrombosis of the renal vein may occur as a result of the hypercoagulable, posttransplant, and postoperative states mentioned in previously. In addition, blunt trauma30 and infection play roles in its development. The most common disease associated with the development of renal vein thrombosis is nephrotic syndrome.27,28 Patients have direct loss of protein S and antithrombin III in their urine.26 With excessive proteinuria, the liver is stimulated to produce new proteins, many of which are prothrombotic.27
Treatment of renal vein thrombosis is generally medical with systemic anticoagulation. Patients with, or at risk for, severe disease (eg, extensive clot progressing to the IVC, renal failure, bilateral disease, renal transplant) may be candidates for systemic or catheter-directed thrombolytic therapy.27
Portal Vein Thrombosis
The estimated lifetime risk of developing portal vein thrombosis in the general population is 1%.31 Up to 15% of cirrhotics develop this condition. It is rarely seen in patients without known liver disease or other risk factors that include adjacent inflammatory conditions (eg, pancreatitis, cholecystitis, diverticulitis, inflammatory bowel disease, appendicitis), malignancies (local or systemic), or hypercoagulable conditions (especially sepsis).32,33 Mortality caused by portal vein thrombosis itself is low; however, these patients frequently have other significant comorbidities that combine to give them poor outcomes with this condition.
The mechanism by which cirrhosis leads to portal vein thrombosis is not clear. It is believed that decreased portal blood flow, periportal inflammation and fibrosis, and impaired production of anticoagulation factors lead to thrombosis.34 Fifty percent of portal vein thrombosis in children and neonates is associated with an intra-abdominal infection, including umbilical infections in the very young.34
With clot in the portal vein, the liver loses approximately two-thirds of its blood supply. In the acute phase, several compensatory mechanisms occur, including dilation of the hepatic artery to increase blood supply, the development of variceal collaterals between the portal and systemic venous systems, and collateral cavernoma formation. The cavernomas are a matted plexus of collateral vessels that form at the porta hepatis, often leading to secondary biliary effects including cholecystitis, biliary obstruction, and jaundice. Although collateral formation ultimately restores some degree of splanchnic circulation, hepatocytes often continue to be underperfused, leading to ongoing ischemia and cell death.32,35
Acute portal vein thrombosis may present with abdominal pain (which may be localized in the right upper quadrant, but is frequently diffuse), nausea, and fever. Signs of intestinal ischemia (discussed later), which is a secondary effect of acute portal vein thrombosis, may also be present.32,34 With chronic thrombosis, patients may remain clinically silent until the secondary effects of the thrombosis occur. These effects include worsening hepatic function, worsening portal hypertension, and hematemesis from esophageal varices.32,34,36
Patients with portal vein thrombosis without complications of bleeding or intestinal ischemia are managed with systemic anticoagulation.31,32,37 Empiric thrombolytic therapy in cases of acute thrombosis may be indicated in severely ill patients in consultation with a gastroenterologist, although there may be significant complication rates.38 Thrombolytics are also considered when standard anticoagulation does not lead to recanalization.32 A transjugular intrahepatic portosystemic shunt (TIPS) procedure may be considered in patients having liver transplants or as an alternative to thrombolytic therapy.
Ischemic bowel
Ischemic bowel can be caused by 1 of 4 mechanisms: arterial thrombosis, arterial embolism, venous thrombosis, or nonocclusive mesenteric ischemia (NOMI). Survival rates vary depending on the mechanism. Overall mortality is as high as 60% to 80%, especially with delays in diagnosis or presentation of greater than 24 hours.39–42 Park and colleagues43 reported worse survival rates in older patients, those not candidates for bowel resection, and those whose cause is NOMI. The overall survival rates for patients with mesenteric ischemia have improved according to a review that analyzed 45 studies and 3692 patients according to cause in almost 4 decades (1966–2002).44,45 Mesenteric ischemia is categorized as occlusive or nonocclusive in origin. Occlusive mesenteric ischemia either involves the SMA or the superior mesenteric vein (SMV). Arterial occlusion may be embolic or thrombotic in origin.
Mesenteric ischemia and ischemic colitis are different clinical entities. The former refers to occlusion of the SMA and SMV, whereas the latter refers to ischemia in the distribution of the IMA. Patients with mesenteric ischemia primarily present with abdominal pain.1,43,46 Patients with ischemic colitis present with lower gastrointestinal bleeding and are less likely to report abdominal pain as the primary complaint. Ischemic colitis has been reported in marathon runners with similar clinical presentations.47 Patients with ischemic colitis tend to be older (77 vs 61 years in a retrospective review of 100 patients presenting to the emergency department [ED]) and show lower overall mortality.48 Angiography is not indicated in cases of ischemic colitis.
Between 40% and 50% of patients with mesenteric ischemia have an arterial embolus as the cause. Emboli typically lodge in the SMA because of the narrow angle it subtends as it branches off the abdominal aorta. Less commonly, emboli lodge in the IMA and, rarely, in the celiac artery.39,46,49 In most cases, a patient with underlying cardiac abnormalities presents with acute onset of pain. Predisposing cardiac conditions include arrhythmia (most commonly atrial fibrillation), myocardial infarction, cardiomyopathy, recent angiography, valvular disorder (eg, rheumatic valve disease), or ventricular aneurysm.42,50,51 Following surgical embolectomy, the mortality from analyzed results from 4 decades is 54%.44
Thrombotic Occlusion of the Mesenteric Artery
Atherosclerosis is the major cause of arterial thrombosis leading to ischemia of the SMA and is the cause of mesenteric ischemia in 25% of patients.39,42 The onset of pain is usually more insidious and may be initially intermittent and eventually becoming constant. Other causative factors are hypercoagulability, estrogen therapy, and prolonged hypotension.50 Patients with chronic ischemia may present with intestinal angina (typically epigastric pain precipitated by eating) and describe food fear and ensuing weight loss.42 Chronic mesenteric ischemia is usually caused by atherosclerosis and is more common in women and smokers. It is also associated with radiation arteritis, autoimmune arteritides, and fibromuscular dysplasia.52 Mortality is high for this disorder and, following surgical treatment, is reportedly 77% (Fig. 3).44
Thrombotic Occlusion of the Mesenteric Vein
Mesenteric venous thrombosis accounts for 10% to 15% of mesenteric ischemia cases. Risk factors for mesenteric venous thrombosis include oral contraceptives or estrogen therapy, malignancy, hypercoagulability, portal hypertension, portal vein thrombosis or other deep vein thrombosis, sickle cell disease, clotting disorders, hepatosplenomegaly, hepatitis, pancreatitis, coagulopathic states, sepsis, cigarette smoking, prior abdominal surgery, and alcohol use.42,50,52–56 Twenty percent of mesenteric venous thrombosis cases are found to be idiopathic. Patients may present in a subacute fashion with abdominal pain and diarrhea. Clinical findings are likely to be more severe, with frank peritonitis and bleeding in patients with extensive transmural ischemia. With chronic mesenteric vein thrombosis, the collateral circulation usually allows for adequate venous drainage, limiting symptoms and consequent secondary effects of portal hypertension and varix formation. Mesenteric venous thrombosis is usually diagnosed by CT with intravenous contrast. This modality has the advantage compared with color Doppler ultrasound of also allowing for assessment of the bowel and other intra-abdominal conditions. Most mesenteric venous ischemia is treated nonoperatively with anticoagulation. As with portal vein thrombosis, a consideration of the harm-to-benefit profile of thrombolytics can be undertaken in consultation with a gastroenterologist as clinical indications dictate. The pooled reported mortality following surgical treatment of venous thrombosis is 32%.44
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree