CHAPTER 26 Abdominal Abscesses and Gastrointestinal Fistulas
ABDOMINAL ABSCESSES
PATHOPHYSIOLOGY
The development of an intra-abdominal abscess (IAA) occurs as a result of a host response to intra-abdominal bacterial contamination secondary to, or in conjunction with, various pathologic clinical entities. In 60% to 80% of cases, intra-abdominal abscess formation is associated with perforated hollow viscera, whether secondary to inflammatory disease such as appendicitis or diverticulitis or as a consequence of penetrating or blunt trauma to the abdomen.1–12 Other conditions associated with IAA formation include inflammatory bowel disease and complications of elective surgery (Table 26-1). Abscesses associated with solid organs such as the pancreas or liver are discussed in Chapters 58, 61, and 82.
Table 26-1 Causes of Intra-abdominal Abscesses
Clinical risk factors for the development of an IAA fall into two general categories: (1) factors related to the intra-abdominal source of infection found at the time of surgery for peritonitis and that can be considered local factors (see Chapter 37); and (2) factors that may have been present prior to surgery (e.g., preexisting comorbidities) or are related to generalized care of the patient during surgery, which can be considered systemic factors. Table 26-2 lists the local and systemic factors associated with increased risk of abscess formation postoperatively.
Table 26-2 Clinical Risk Factors for Intra-abdominal Abscess Formation
| Systemic Factors |
| Local Factors |
There is a delicate balance of opposing forces in the peritoneal cavity between bacterial factors and the host’s defense mechanisms, which attempt to clear bacterial contamination and localize infection (Table 26-3). These two opposing forces are often influenced by the presence of adjuvant factors (e.g., foreign material, fibrin) in the peritoneal cavity that often tip the balance toward bacterial infection with abscess formation (see Chapter 37).
Table 26-3 Factors Influencing Transition from Bacterial Contamination to Infection
| BACTERIAL FACTORS | ADJUVANT FACTORS | HOST DEFENSE FACTORS |
|---|---|---|
Adapted from Fartham EH, Schoffel U. Epidemiology and pathophysiology of intra-abdominal infections (IAI). Infection 1998; 26:329-34.
Once bacteria gain access to the peritoneal cavity through perforation of the intestinal wall, several factors come into play that determine whether an active infection is initiated. The typical bacteria that make up intra-abdominal infections have the ability to adhere to peritoneal surfaces and selectively grow and use host nutrients. These bacteria can undergo metabolic processes that are adapted to the host environment (e.g., obligate anaerobic metabolism). Furthermore, these bacteria have the capacity to resist antibiotic attack. Bacterial synergy plays an important role in the development of intra-abdominal infection (see later, “Bacteriology”).6
The peritoneum uses a number of host defenses to combat bacterial contamination.6,13 The balance of host defense factors in the setting of adjuvant factors determines whether contamination continues on to infection. Lymphatic clearance of bacteria is a major defense process that is so efficient that abscess formation occurs only when adjuvant substances such as hemoglobin, barium, or necrotic tissue are present.14 These adjuvant substances may block lymphatics (barium, fecal particulate matter), provide bacterial nutrients (iron from hemoglobin), or impair bacterial killing. Shortly after bacterial contamination, peritoneal macrophages are the predominant phagocytic cells. These cells are also cleared by the lymphatic system. As bacteria proliferate, polymorphonuclear leukocytes invade and become more numerous. The resultant peritoneal inflammation leads to an increase in splanchnic blood flow, with protein and fluid exudation into the peritoneal cavity. Procoagulatory effects of the inflammatory process and reduced levels of plasminogen activator activity enhance fibrin deposition and lead to entrapment of bacteria and localization of infection.13
These peritoneal defense mechanisms can have adverse effects. Lymphatic clearance of bacteria may be so brisk and effective that it results in a systemic response to bacteremia and sepsis. The exudation of fluid into the peritoneal cavity can lead to hypovolemia and shock; it can also dilute the opsonins needed in phagocytosis. Fibrin entrapment of bacteria can impair antimicrobial penetration and phagocytic migration with the potential to localize infection and lead to abscess formation.13 However, attempts to alter this balance of defense mechanisms are still not fully understood. In a study using a rodent intraperitoneal abscess model, recombinant tissue plasminogen activator (rt-PA) was used to increase intra-abdominal fibrinolytic activity. Rats treated with rt-PA had significantly fewer abscesses than controls, but they had significantly more bacteremic episodes and higher mortality rates.15 Further work in this area by the same group used similar rodent models of intraperitoneal infection to study the role of a hyaluronic acid solution in abdominal adhesion and abscess formation. In bacterial peritonitis, intraperitoneal hyaluronic acid solution in the presence of antibiotics reduced the development of adhesions and abscess formation without increasing mortality.16 Possible mechanisms of action include mechanical separation of wound surfaces, improvement of peritoneal healing, modulation of the inflammatory response, and enhanced fibrinolysis.17 The potential of hyaluronate-based agents to reduce intra-abdominal adhesions and abscesses in abdominal surgery and sepsis is a promising new concept.
Studies have suggested that the formation of adhesions is a complicated process that is not only dependent on surface apposition but is also under the tight control of positive and negative T cell costimulation.18 This was exemplified by a clinical trial19 of more than 1700 patients undergoing abdominopelvic surgery of the intestine, most for complications of inflammatory bowel disease. Patients were randomized to an adhesion barrier (Seprafilm [modified sodium hyaluronic acid and carboxymethyl cellulose], Genzyme, Cambridge, Mass) or control (no intervention) placed at the time of abdominal closing. Although abdominal and pelvic abscess rates were not different between the groups, there was a statistically higher rate of postoperative fistula formation and peritonitis in the Seprafilm group. This was particularly noted in patients who had the adhesion barrier wrapped around fresh intestinal anastomoses. Extensive experience has now accumulated with the use of Seprafilm. A recent meta-analysis20 of eight studies and over 4000 patients has shown that Seprafilm clearly decreases the severity and extent of postoperative adhesions. However, this came at the cost of increased rates of abdominal abscesses and anastomotic leaks. The effect was enhanced in the setting of surgery for inflammatory bowel disease. Newer preparations of hyaluronate-based membranes have also been associated with increased formation of postoperative intra-abdominal abscesses.21 Thus, the evidence for Seprafilm increasing postoperative abscess formation after surgery for acute peritonitis appears to be growing. The use of Seprafilm in this clinical situation to reduce postoperative abscess formation cannot be recommended.
BACTERIOLOGY
The typical abscess that forms as a complication of secondary bacterial peritonitis, defined as loss of integrity of the gastrointestinal (GI) tract, is a mixed aerobic and anaerobic infection. In studies of isolates from subphrenic,22 retroperitoneal,23 and diverticular abscesses,24 a range of 2.9 to 3.7 bacterial isolates per abscess was recovered. The most common aerobes were Escherichia coli and Enterococcus species (range, 1.3 to 1.6 isolates per specimen). The most common anaerobes were Bacteroides fragilis and Peptostreptococcus species, which accounted for 50% to 75% of all anaerobes isolated. Other Bacteroides species and Clostridium species made up the remainder of anaerobes isolated (range, 1.7 to 2.1 isolates per specimen). In all three studies, most abscesses contained mixed aerobic and anaerobic flora (60% to 75%); the minority contained aerobic isolates only (10% to 20%) or anaerobic isolates only (15% to 20%). The number of anaerobic isolates always was higher than the number of aerobic isolates.
Bacteroides species are important microbes in the formation of IAA. The existence of specific repeating negatively and positively charged cell wall polysaccharides on B. fragilis leads to a host response that results in the formation of an IAA. This host response is T cell–mediated and abscess formation can be experimentally prevented by vaccination with these repeating polysaccharide units. This vaccination does not appear to be antigen-specific in the traditional sense. Rather, the protective ability of these polysaccharides is conferred by, and perhaps specific for, a motif of oppositely charged groups. Vaccination with B. fragilis capsular polysaccharide complex significantly reduced the mortality rate and intra-abdominal abscess formation in a rat cecal ligation and puncture model.25 The cellular mechanism of IAA formation by B. fragilis has been elucidated.25 B. fragilis capsular polysaccharide complex adheres to peritoneal mesothelial cells and interacts with T cells and peritoneal macrophages to produce proinflammatory cytokines and chemokines, with subsequent expression of intercellular adhesion molecule-1 (ICAM-1) on host cells and recruitment of polymorphonuclear leukocytes to the abdominal cavity. Thus, the role of the capsular polysaccharide complex is to promote adhesion of B. fragilis to the peritoneal wall and coordinate the cellular events leading to the development of abscesses.
The bacteria associated with intra-abdominal infections and abscesses in patients in the ICU who have been subjected to broad-spectrum antimicrobial selection pressure are different from those in patients with abscesses that result from secondary bacterial peritonitis. Thus, the microbiologic agents that cause tertiary peritonitis, defined as persistent intra-abdominal sepsis with or without a discrete focus of infection, generally after an operation for secondary peritonitis, are no longer E. coli and B. fragilis (see Chapter 37). Rather, nosocomial infections with resistant gram-negative organisms, Enterococcus species, and/or yeast are more common.26,27 The microbiologic analysis of abscesses in severely ill patients (Acute Physiology and Chronic Health Evaluation [APACHE] II score > 15) revealed that 38% had monomicrobial infections. The most common organisms were Candida (41%), Enterococcus (31%), and Enterobacter (21%) spp. and Staphylococcus epidermidis (21%); E. coli and Bacteroides spp. accounted for only 17% and 7%, respectively.28
DIAGNOSIS AND TREATMENT
The optimal management of the patient with an IAA includes the following: (1) accurate diagnosis and localization of the collection; (2) removal or control of the source of peritoneal contamination; (3) drainage of any established collections; (4) elimination of residual contamination of the peritoneum through antimicrobial therapy; and (5) physiologic support of the patient.13 The symptoms and signs of IAA are nonspecific, and a high level of vigilance is needed to make the diagnosis. Fever and elevated leukocyte count are frequent but nonspecific findings. Abdominal pain, tenderness to palpation, distention, and a palpable mass are also common findings. Suspicion of the presence of an IAA warrants further diagnostic imaging.
Diagnostic Imaging
Computed Tomography
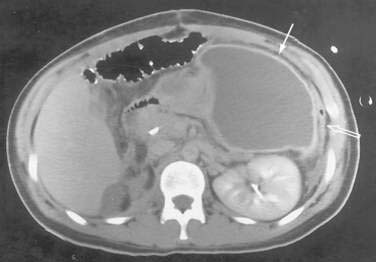
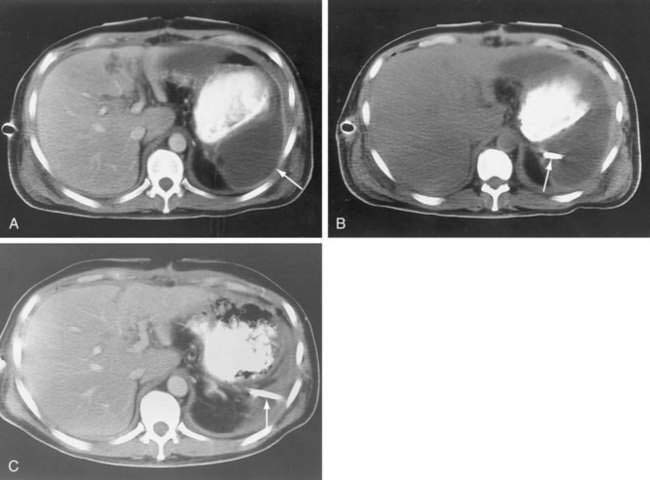
The CT diagnosis of abdominal abscess is suggested by identification of a loculated fluid density in an extraluminal location. Extraluminal gas within an abdominal mass is highly suggestive of an abscess, although necrotic tumors and resolving hematomas may occasionally exhibit this finding. Wall enhancement and adjacent inflammation favor the likelihood of infection in fluid collections (Fig. 26-1). Any fluid collection on CT should be clearly differentiated from nonopacified bowel. Delayed images are often necessary to allow bowel to opacify fully and to allow the investigator to distinguish an abscess from bowel confidently. The fluid in an abscess may occasionally be higher in density when proteinaceous material is present or when the collection represents an infected hematoma. Phlegmonous inflammatory tissue does not exhibit fluid density; rather, it is solid in appearance, often with inhomogeneous enhancement.
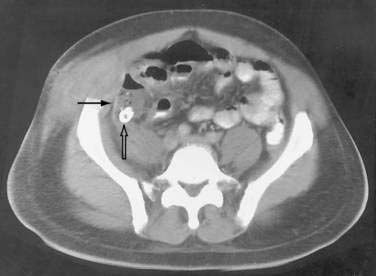
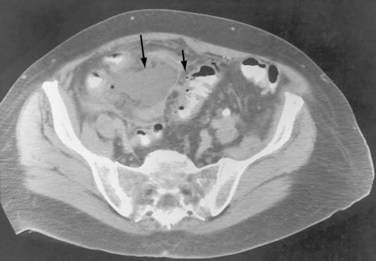
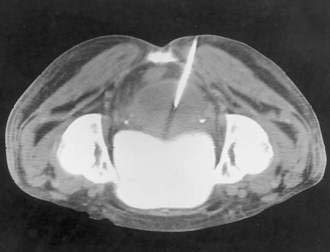
In some cases, the CT appearance can suggest the cause of the abscess. Periappendiceal abscesses commonly have a characteristic location in the right lower quadrant adjacent to the cecum and may demonstrate an appendicolith (Fig. 26-2). Peridiverticular abscesses are often associated with an inflamed adjacent colon demonstrating diverticula (Fig. 26-3). Abscesses associated with Crohn’s disease may demonstrate adjacent thickened small bowel.
Ultrasonography
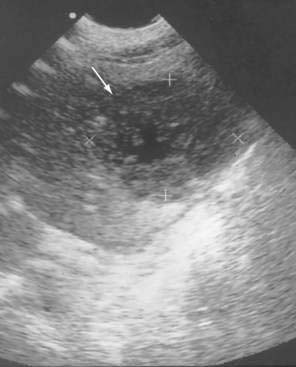
The classic ultrasonographic appearance of an abscess is a localized rounded or oval area of decreased echogenicity with internal debris and a thick irregular wall (Fig. 26-4). Most abscesses exhibit fluid characteristics on ultrasonography, but some may appear solid as a result of thick debris. Internal septations may be seen and are better identified by ultrasound than by CT. Gas within an abscess is suggested when areas of increased echogenicity are present, with posterior shadowing. The shadowing behind a gas collection tends to be less distinct than the more defined shadowing identified behind calculi on ultrasound. There is considerable overlap of the ultrasonographic appearance of infected and sterile fluid collections, and diagnostic aspiration is necessary to differentiate them. Ultrasound can be used for guidance during some percutaneous drainage procedures; however, poor visualization of intervening structures such as bowel in the midabdomen may limit its usefulness in some anatomic areas. Superficial and large abscesses tend to be more amenable to ultrasound guidance than smaller and deeper abscesses.
Mode of Drainage
Once an IAA is diagnosed and localized, a decision must be made regarding the optimal drainage technique and route. Percutaneous abscess drainage (PAD) has been shown to have equivalent success rates and less risk compared with surgery,29 although no randomized prospective studies are available. Assuming the availability of a safe route to the abscess, as occurs in 85% to 90% of cases, PAD should be the drainage procedure of choice.30–36 In the past, multiloculated, poorly organized, and multiple abscesses were not considered good candidates for PAD because of higher failure rates in these cases. Indications for PAD have now broadened to include these more challenging circumstances in many institutions, although longer drainage duration and multiple interventions may be necessary to obtain success.30–37 Although abscesses associated with enteric fistulae have lower success rates with PAD, successful PAD can be achieved in many cases.31,38 Rates of spontaneous closure up to 57% have been reported when aggressive catheter management has been combined with nutritional support.39 Interloop or intramesenteric collections are often not accessible percutaneously and surgery is often necessary. PAD is not appropriate for uncontained perforations or diffuse peritonitis.31,32,35 If surgery is chosen as the drainage mode of IAA (see later), an extraperitoneal approach is desirable to prevent contamination of the entire abdominal cavity.40 When feasible, some pancreatic abscesses or walled-off pancreatic necrosis (WOPN) can be drained endoscopically (see Chapter 61).
Percutaneous Abscess Drainage
Continuing advances in diagnostic imaging and percutaneous catheter development have allowed PAD of abdominal abscesses combined with systemic antibiotic therapy to become the standard initial treatment of abdominal abscesses.30–36,41,42 Success rates for PAD range from 70% to 93%.29,31,33,36–38,42 Most abdominal and pelvic abscesses can be safely accessed percutaneously. A safe route into the abscess should be chosen that avoids major vascular structures, bowel, and adjacent organs. In extreme circumstances, the liver and the stomach can be traversed.35 Small and large bowel should not be traversed with a catheter, making interloop abscesses often inaccessible percutaneously. With increasing experience of interventional radiologists, indications for PAD have expanded to include multiple abscesses, multiloculated abscesses, poorly defined collections, and more challenging access routes.30–36,38,43 Adjunctive thrombolytics can be used safely to increase success in septated abscesses or when thick debris is encountered.44
An inflammatory phlegmon without demonstrable fluid collection is not appropriate for percutaneous drainage. Some small fluid collections, typically less than 3 cm, also may not require a catheter and can be managed through percutaneous aspiration for diagnosis, followed by antibiotic therapy. Catheter management is generally preferred for larger collections in most institutions; however, one-step percutaneous needle aspiration of abdominal and pelvic abscesses combined with systemic antibiotics has also been advocated as an alternative to catheter placement in larger abscesses.45 Contraindications to PAD include lack of a safe access route and uncorrectable coagulopathy.32 Coagulation studies and correction of any coagulopathy are recommended before the procedure to reduce the risk of uncontrollable hemorrhage.
Guidance for PAD can be accomplished with a variety of imaging modalities including CT, ultrasonography, and fluoroscopy, or a combination of modalities. The imaging modality selected is dependent on the location and size of the abscess as well as operator preference. The most common imaging modality is CT because of its widespread use for the initial diagnosis of abdominal abscess and its superb visualization of bowel and vascular anatomy. Ultrasonography can provide more real-time visualization during catheter insertion and can be useful when extreme angling of the route is needed.34,35
After a safe percutaneous route is identified, the cavity is accessed using a trocar method or a needle and guidewire method. The tract is then dilated to a diameter approximating that of the planned catheter, and the catheter is advanced into the cavity. A sump-type double-lumen catheter is the most common catheter used. A 12- or 14-Fr catheter size is generally adequate to drain most abscesses, although a larger catheter size may be necessary for an abscess associated with a large amount of debris or hemorrhage.38
Postdrainage Management
PAD endpoints and decisions to obtain follow-up imaging studies depend on the clinical response, catheter drainage, and presence of suspected enteric communications. If the clinical response has been satisfactory and the catheter drainage has diminished to less than 20 mL/day, the catheter can be safely removed. If clinical response is inadequate, repeat imaging is warranted. Persistently high catheter output raises suspicion of a fistula. A catheter study performed by instilling water-soluble contrast medium through the catheter under fluoroscopy is the best method to assess for an internal fistula as the cause of high drainage output. If a fistula is located, the catheter can be repositioned adjacent to the opening into the bowel for better control of bowel effluent. Poor clinical response can also be caused by catheter dislodgment from the major abscess cavity, undrained loculations, multiple abscesses, or new abscesses. Repeat CT can evaluate for these possible causes of poor clinical response and guide additional percutaneous interventions when appropriate. Thick debris may occlude the catheter and inhibit daily flushing. In this case, the catheter can be exchanged for a larger catheter.41,46
Complications of Percutaneous Abscess Drainage
The complication rate of PAD ranges from 4% to 15%.29,38,41,47 Complications include transient sepsis, organ injury, hemorrhage, pneumothorax, peritonitis, empyema, and pain.
Recurrent Abscesses
Intra-abdominal abscess recurrence rates range from 1% to 9%.29,31,41,48 Even when an abscess recurs, repeat secondary PAD should be considered and can be curative. Success rates for secondary PAD up to 91% have been achieved in recurrent abscesses, although the mean duration of drainage to achieve success was significantly longer with the secondary procedure.31
Drainage of Specific Abscesses
Subphrenic Abscesses
Subphrenic abscesses can be drained percutaneously, with careful attention to technique (Fig. 26-5). Avoidance of the pleural space is optimal to prevent pneumothorax and seeding of infection to the chest. The pleural space typically extends to the level of the eighth thoracic vertebra (T8) anteriorly, T10 laterally, and T12 posteriorly. These guidelines can be used to prevent traversing of the pleural space. Some subphrenic fluid collections may not allow an extrapleural approach, in which case surgical risks should be weighed against the increased risk of empyema and pneumothorax posed by transpleural PAD. The safety of a transpleural approach has been debated.48,49
Pelvic Abscesses
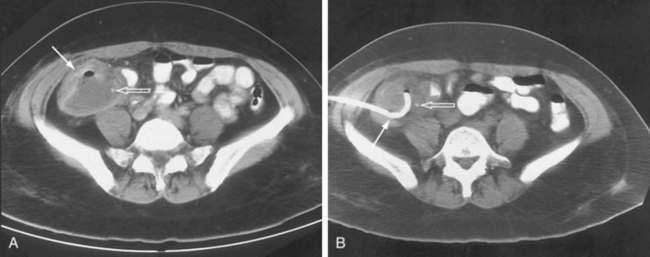
Anterior access to pelvic abscesses can be limited by intervening bowel, bladder, uterus, or vascular structures. A posterior transgluteal approach through the sciatic notch50 with the patient in the prone position has been used to drain deep pelvic fluid collections that are not accessible to an anterior approach (Fig. 26-6). Care must be taken to avoid the gluteal vasculature and the sciatic nerve. Ultrasound-guided transvaginal and transrectal drainage techniques have also been increasingly used for drainage of deep pelvic abscesses that are not accessible through other routes.51,52 A comparison of transrectal and transvaginal techniques has demonstrated better patient tolerance of the transrectal drainage route.53
Appendiceal Abscesses
Periappendiceal abscesses can often be suggested by the CT appearance (Fig. 26-7A; see Fig. 26-2). Percutaneous abscess drainage has been increasingly accepted as the initial management of sepsis associated with a periappendiceal abscess, allowing the surgeon to perform a subsequent appendectomy, often laparoscopically, on an elective basis (see Fig. 26-7B).54,55
Surgical Management
Surgical management of an infected patient is indicated when the patient is not a candidate for PAD secondary to multiple or interloop abscesses, or inability to access the cavity. Alternatively, surgery may be indicated for failure of PAD to control the source of infection. In these cases, care must be taken to adequately plan the route for surgical abscess drainage adequately. If possible, an extraperitoneal approach, such as the posterior approach to a left subphrenic abscess with 12th rib resection,57 will allow dependent drainage of the collection without contaminating the remainder of the peritoneal cavity.
There are two groups of patients who pose difficult surgical challenges. These patients have an overwhelming intra-abdominal infection noted at their first operation with significant bowel inflammation and edema,58 or they have failed initial therapy aimed at controlling secondary peritonitis and are now being managed for tertiary peritonitis.59,60 In both cases, these patients will often be treated with an open abdomen. Simply defined, the term open abdomen
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree