Fig. 17.1
PET scan demonstrating too many lesions to be able to safely and effectively perform ablation with curative intent
Tumors which are 3 cm in size require the creation of a 5 cm ablation zone. While RFA and MWA ablation technologies can create reliable ablation zones up to this size, larger zones become progressively more unreliable and dangerous to central portal structures. Local treatment failure rates for tumors less than 3 cm in size are typically well below 10 % but increase sharply above this size [9]. Similarly, tumors of any size can be ablated using chemical infiltration, but the larger the lesion, the less likely the cell kill will be 100 %. This problem can be overcome, to some degree, by using multiple ablation sessions. However, recurrence rates following ethanol injection for tumors larger than 3 cm are often reported to be above 20 % [13].
Operating Room Setup
Whether using open or laparoscopic techniques, the optimal setup for surgical ultrasound-guided tumor ablation is to have the surgeon and the ultrasound image on opposite sides of the target tumor. For liver tumor ablations, this typically involves the surgeon standing on the patient’s left side, about hip level. The ultrasound monitor (and laparoscopic monitor) is on the patient’s right side, usually about shoulder level. This allows the surgeon to comfortably look directly across the table, with the surgeon’s line of sight in alignment with the target tumor and imaging displays. This will facilitate the surgeon’s ability to create a three-dimensional mental image of the target and optimize the approach with an ablative probe (Fig. 17.2a).
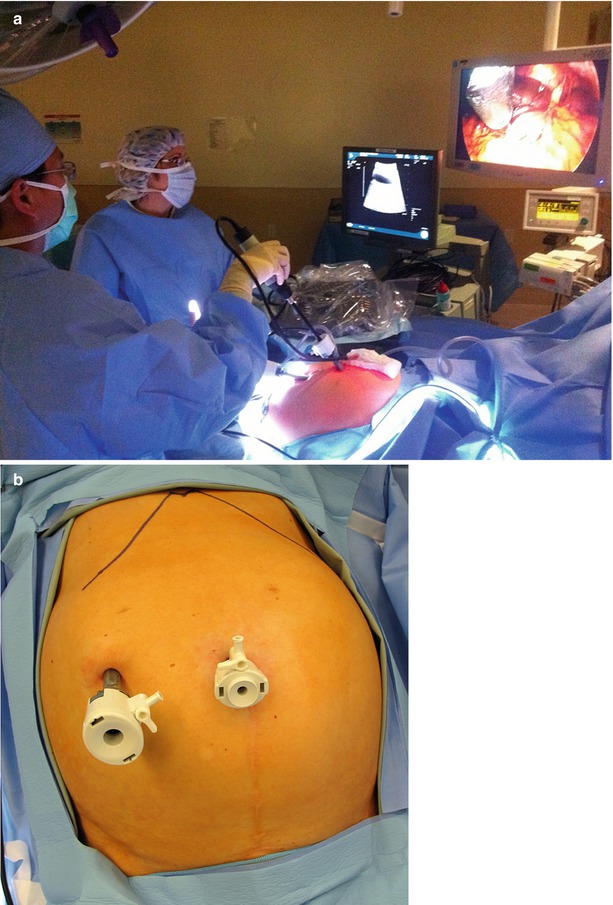

Fig. 17.2
(a) Operating room with the surgeon standing on the patient’s left and the US and laparoscopy monitors diametrically opposite the liver. (b) Inset demonstrates typical port positions for a staging laparoscopy, intraoperative US, and tumor ablation
Laparoscopic Staging
Laparoscopic staging includes a thorough peritoneoscopy. Specifics of the case will determine the degree to which visceral manipulation will be required to assess the possibility of extrahepatic disease. For patients with colorectal liver metastases who have a high-quality CT scan and are otherwise at low risk for intra-abdominal recurrence, we use a two-port technique (Fig. 17.2b). We start by viewing the easily visible, non-hepatic surfaces of the peritoneal cavity with little visceral manipulation. If there are specific concerns for extrahepatic recurrence, we will add additional ports to mobilize the abdominal viscera sufficiently to explore the entire abdominal and pelvic cavities.
The liver surface is scanned visually. Adhesiolysis is performed as necessary to visualize the anterior surface and dome of the liver. Visualization of the under surface of the liver is facilitated using an instrument through the second port. The falciform, triangular, and coronary ligaments are divided selectively, as it is usually possible to perform through ultrasound examination of the liver without doing so. The porta hepatis may be visualized with ultrasound through segment 4 of the liver (Fig. 17.3a) or by direct contact through the hepatoduodenal ligament (Fig. 17.3b). Identification of portal, peripancreatic, or para-celiac lymphadenopathy may alter the intended treatment strategy, particularly in cases when extrahepatic pathology is a contraindication to liver-directed therapies (Fig. 17.4).
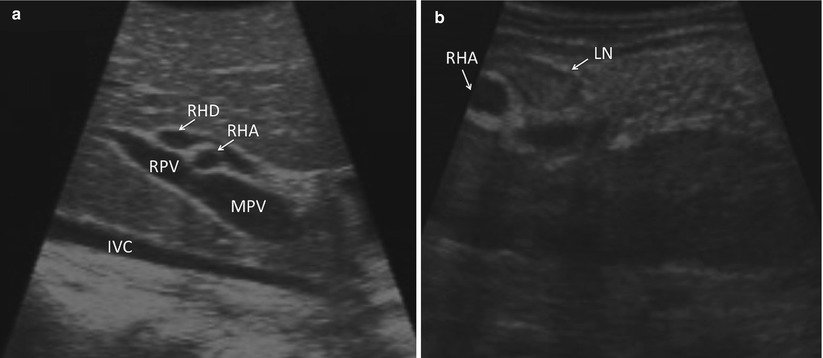
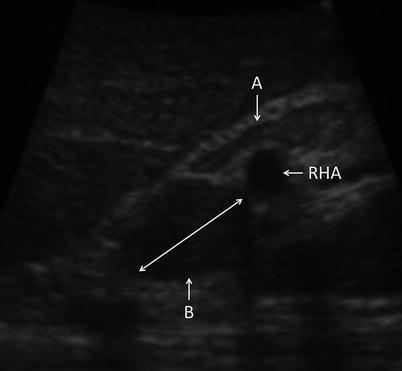

Fig. 17.3
(a) US demonstrates an image of the porta hepatis as seen when scanned through segment 4b of the liver (IVC inferior vena cava, MPV main portal vein, RPV right portal vein, RHA right hepatic artery, RHD right hepatic duct). (b) US demonstrates an image of the porta as seen with direct application of the US probe to the porta hepatis (RHA right hepatic artery, LN lymph node)

Fig. 17.4
US image demonstrating portal lymph nodes. Node A is normal. Node B is infiltrated with tumor (RHA right hepatic artery)
Biopsies are obtained at the discretion of the surgeon. Unexpected findings in the liver, such as fibrosis or cirrhosis, may be of importance in planning the ablation or in the planning of future medical management. Wedge or core needle biopsies of non-tumoral liver tissue may be obtained for pathologic analysis. Unexpected lesions within the liver are biopsied if there is a question regarding their etiology. Wedge biopsies of surface lesions and core needle biopsies penetrating directly into surface lesions are discouraged as they may result in tumor seeding of the peritoneal cavity. Core biopsies are best obtained by transiting a minimum of 1 cm of normal liver parenchyma prior to entering the target tumor. This will reduce the risks associated with tumor spillage (Fig. 17.5).
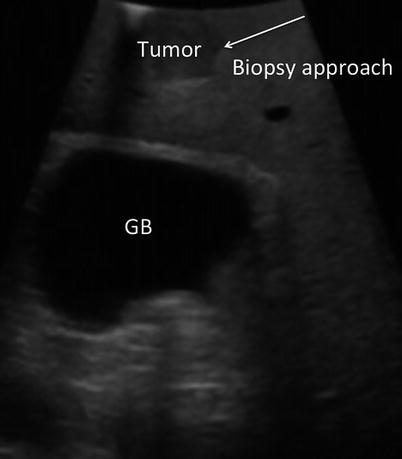

Fig. 17.5
US image demonstrates a tumor near the surface of the liver. Biopsy of surface lesions should be performed via an angle that allows transit of at least 1 cm of normal tissue prior to penetrating tumor. This approach will reduce the risk of peritoneal seeding of the tumor (GB gallbladder)
Surgical Approach
Like the difference between tumor ablation and liver resection, a laparoscopic or open approach should not be considered universally applicable. Each of these approaches is a tool to be used as needed by knowledgeable and skillful surgeons. A laparoscopic approach has the advantages of being less invasive. This has been clearly demonstrated to yield lower perioperative morbidity and mortality rates and allow faster recovery. Faster recovery is especially important considering that many patients have a poor prognosis and a short anticipated time of survival. One practical limitation of the laparoscopic approach is that many surgeons have not had adequate training to feel fully competent in the technique of laparoscopic ultrasound or the application of laparoscopic ablation. While there are no areas of the liver that cannot be accessed using laparoscopic techniques, addressing lesions in segments 4a, 7, and 8 can be challenging. These may require advanced laparoscopic techniques not currently universally available.
The advantages of an open approach include the ability to add a manual component to the staging procedure. There is no surgical instrument as refined or sensitive as the human hand at identifying subtle changes in texture or firmness, which may be indications of additional pathology. The other primary utilization of an open ablative approach is in combination with open surgical resection. Some tumor burdens, for example, may require a combination of a formal left or right hepatectomy and ablation of one or more lesions in the contralateral, remnant liver.
Liver Mapping
Prior to initiation of liver mapping, it is important to make sure that the ultrasound is optimized for the conditions at hand. Optimizing ultrasound settings and technique for the liver are covered in Chaps. 4 and 15. In general, it is best to use the highest frequency that penetrates to adequate depths within the liver. For most livers, 10 MHz is appropriate. For fatty or cirrhotic livers, 5–7.5 MHz may allow better imaging of deeper structures. Familiarity with Doppler features is occasionally helpful in differentiating between vascular and biliary structures or determining whether there is flow in a vessel.
It is important to perform a thorough screening of the liver at the time of ablation. This is done by locating key structures within the liver, dividing the liver into known anatomic sections/sectors and segments, and screening each in a standardized fashion. Using this regimented approach, additional lesions not seen on preoperative imaging may be found in 10–25 % of cases [14–16]. Intraoperative ultrasound may identify lesions as small as 3 mm. Although it may be difficult to accurately biopsy such lesions, knowledge of their presence may significantly alter the course of the operation.
Using the Brisbane terminology [17], the liver is divided into sections (or sectors) using the hepatic veins. We start by advancing the ultrasound probe to the diaphragm on the patient’s right side of the falciform ligament, where the vena cava and the orifices of the left, middle, and right hepatic veins are identified (Fig. 17.6a, b). The middle hepatic vein divides the liver into left and right livers. The right hepatic vein divides the right liver into the right anterior section/sector and the right posterior section/sector. The left hepatic vein is used to divide the left liver into medial (segments 3 and 4) and lateral (segment 2) sections. For clarification, it should be noted that many surgeons divide the left liver into a left medial sector (segment 4) and a left lateral sector (segments 2 and 3) [18].
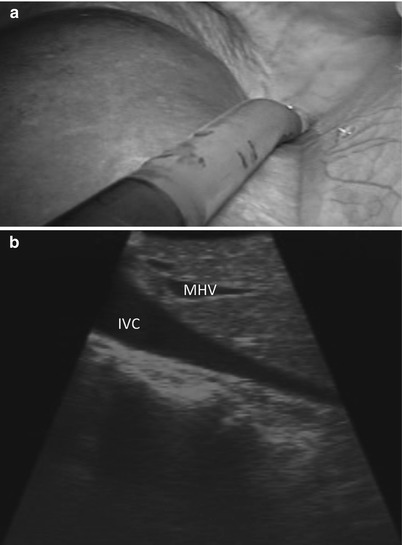

Fig. 17.6
(a) Laparoscopic view demonstrates the tip of the US probe over segment 4a, from which the inferior vena cava and the base of the hepatic veins can be identified. (b) US image demonstrates the inferior vena cava (IVC) and middle hepatic vein (MHV). The MHV can be identified as it empties into the IVC near the toe of the US probe and runs toward the heel of the US probe. The left and right hepatic veins can be viewed by rolling the probe to the patient’s left and right, respectively
With the sections/sectors demarcated, the ultrasound probe is drawn back until the portal bifurcation is identified (Fig. 17.7). Sweeping left and right, the branches of the left and right portal veins can be followed to locate the individual segments of the liver. Each segment can then be screened individually for lesions. Thorough screening of the liver requires a combination of two different types of motion with the ultrasound probe. A sweeping motion is used to move across the surface of the liver (Fig. 17.8a). This motion is used to move from segment to segment and to scan immediately under the surface of the liver. A side-to-side rolling motion is used to scan within the parenchyma of each segment (Fig. 17.8b). This allows a more careful examination of deeper tissues. It is important to keep the shaft of the ultrasound straight during the rolling motion. The combination of these two probe maneuvers is required to assure a complete evaluation of all hepatic tissue (Fig. 17.8c, d). (Refer to Chap. 4 for further information.)
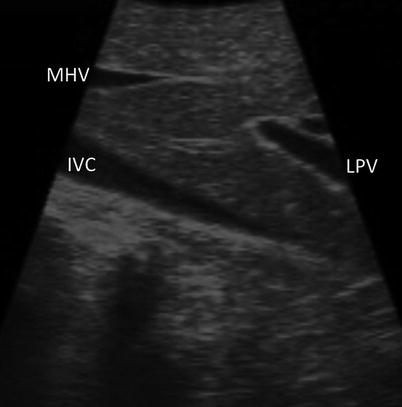
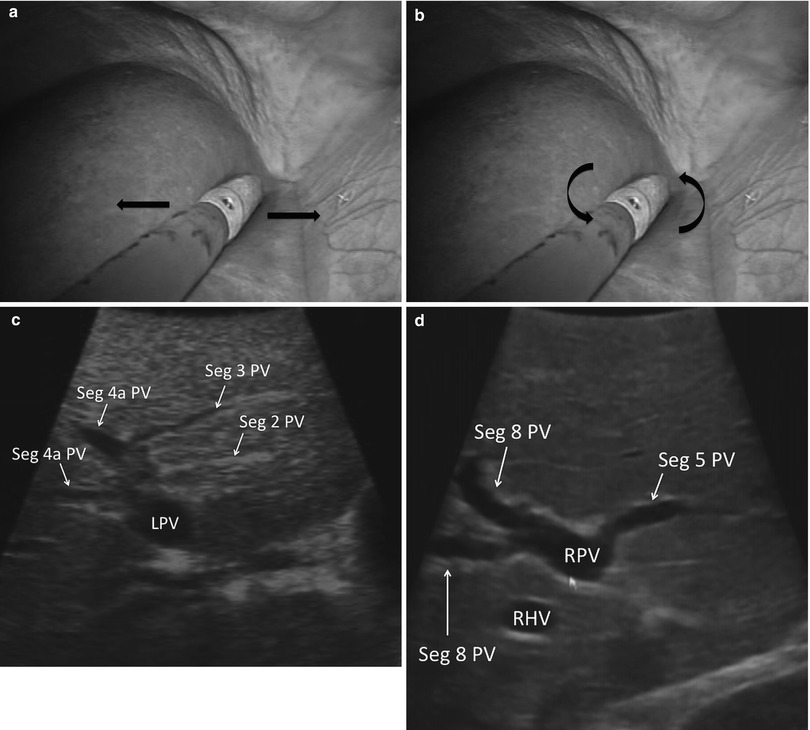

Fig. 17.7
US image demonstrating that the LPV (LPV, portal vein) is seen when the US probe is drawn straight back from segment 4a (MHV middle hepatic vein, IVC inferior vena cava)

Fig. 17.8
(a) Laparoscopic image showing the US probe sweeping left to right. (b) Laparoscopic image showing the US probe rolling. Note the shaft of the US probe is straight. (c) US image demonstrating the segmental anatomy of the left liver (LPV left portal vein, Seg segment, PV portal vein). (d) US image demonstrating the segmental anatomy of the right liver anterior section (RPV right portal vein, Seg segment, PV portal vein, RHV Right Hepatic Vein)
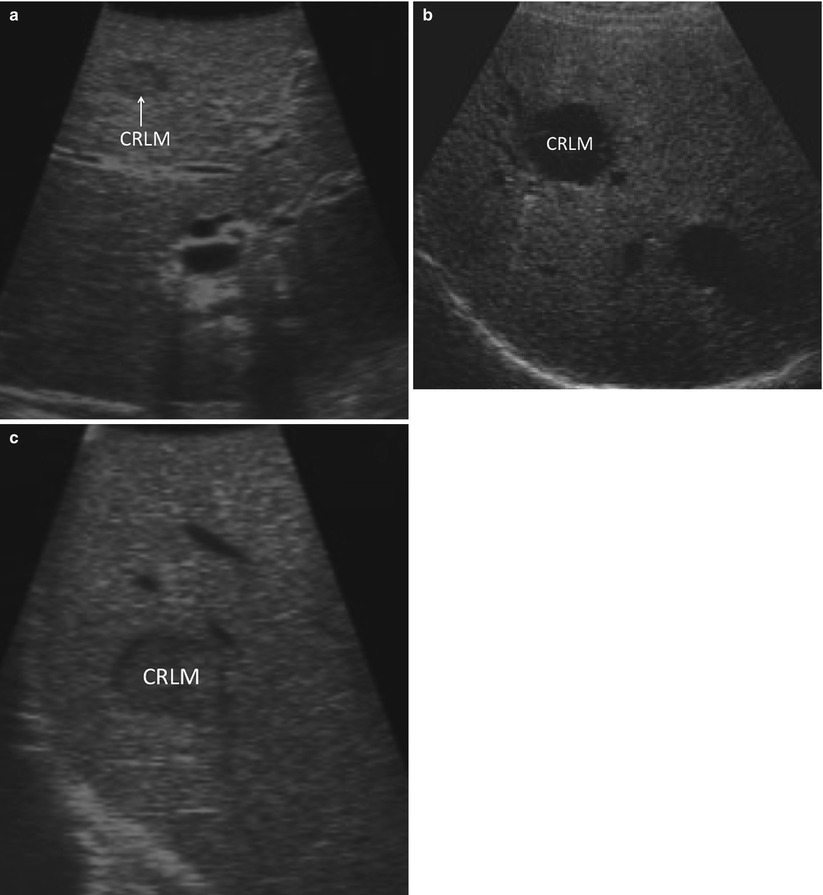
If a targeted lesion is identified early in the screening process, it is helpful to note its ultrasound characteristics. Some tumors demonstrate characteristic morphologic features. For example, metastatic adenocarcinomas tend to have a hypoechoic halo surrounding a heterogeneous, hyperechoic center (Fig. 17.9) [19]. In practice, however, there is a great deal of variability in how lesions appear on ultrasound. Once a known lesion’s ultrasound characteristics are evaluated, it is easier to know what to look for during the remainder of the staging process. Metastatic lesions within an individual patient tend to have a similar appearance.