First author
Location
Age range
Time period studied
Incidence
Henderson 2012 [6]
Scotland
< 16 years
2003–2008
2.1
Benchimol 2009 [7]
Ontario, Canada
< 18 years
1994–2005
1994
4.1
2005
4.2
Adamiak 2013 [8]
Wisconsin, USA
< 18 years
2000–2007
2.4
Jakobsen 2011 [9]
Eastern Denmark, Funen, Aarhus County, Denmark
< 15 years
2007–2009
3.1
Perminow 2009 [10]
Southeastern Norway
< 18 years
2005–2007
3.6
Malmborg 2013 [11]
Northern Stockholm County, Sweden
< 16 years
2002–2007
2.8
Adult studies demonstrate that UC is more prevalent in North America, the UK, and Scandinavia and less common in southern Europe, Asia, and Africa [2]. The data suggest a north-to-south gradient with higher incidence rates of both CD and UC in northern locations, even within individual countries [13–15]. UC is more common among Jewish than non-Jewish peoples [16], but disease rates in people of Jewish origin vary by geographic region and parallel those of the general population [17]. The higher rates of IBD in individuals of Jewish origin across different countries support a common genetic predisposition; however, the geographic variation of IBD rates in Jews emphasizes that environmental factors (see below) influence the inherited risk .
Pathogenesis
No single cause of UC has been identified . Most likely, the disease results from a combination of the interplay of genetic, environmental, and immunologic factors. A widely accepted hypothesis suggests that in the genetically susceptible individual, a combination of host and environmental factors leads to the initiation and perpetuation of an abnormal intestinal immune response to gut flora, resulting in UC [18, 19]. In support of this theory, colitis in animals occurs in a wide variety of genetically altered rodents, including interleukin (IL)-2, IL-10, and T cell receptor knockout mice, and HLA-B27 transgenic rats [20]. Interestingly, most of these animal models do not develop colitis in germ-free environments. These findings suggest that multiple genes may contribute to the pathogenesis of IBD, and that interaction with the environment is essential .
Human Genetics
In humans, UC appears to have a non-Mendelian pattern of inheritance. Current evidence suggests that genes contribute less to the risk in individuals with UC than in those with CD. In a Danish twin cohort study and a Swedish twin cohort study, the calculated pair concordance rates among monozygotic twins were 14–19 % for UC and 50 % for CD, and among dizygotic twins, 0–5 % for UC and 0–4 % for CD [21, 22]. Although the concordance rates of disease are higher in monozygotic twins, the incomplete concordance suggests that nongenetic factors also contribute to the development of UC. First-degree relatives of patients with UC have a 9.5-fold increase in risk of developing UC, compared to the general population [23]. Genome-wide association (GWA) studies have implicated over 100 genes in the pathogenesis of IBD: Some of these genes exclusively predispose to CD, others to UC, and others to both diseases [24].
Environment
Environmental factors may contribute to the development of UC. One mechanism of action by which environment may trigger IBD is by altering the microbiome . Pediatric studies demonstrate that the intestinal microbiota in children with IBD differs from that of the patients with non-IBD gastrointestinal conditions [27, 28]. However, it is difficult to ascertain whether the microbiome alterations cause the intestinal inflammation, or are epiphenomena. Nevertheless, there is good evidence that diet and other environmental factors may alter the microbiome in both animals and humans [29]. Most studies examining environmental risk factors have the limitations of retrospective, case-control methodology. One of the most consistent findings in multiple studies is the lower risk of UC among current smokers. Current smokers have approximately one half the risk of developing UC compared to nonsmokers [30, 31] .
Several authors have suggested that exposure to infections in the perinatal period or early life may contribute to the development of UC. Individuals with CD or UC were more likely to have experienced a diarrheal illness during infancy, when compared to their unaffected siblings [32]. Additional studies suggest associations between infectious gastroenteritis and development of pediatric or adult-onset UC [33–35]. Antibiotic usage in early childhood increases the risk of developing IBD, but the risk goes down as children grow older [36]. Appendectomy at a young age is associated with a lower risk of UC, but it may be the underlying appendiceal infection rather than the appendectomy itself that is the protective factor [37–39].
Other environmental factors that may affect the incidence of colitis include breastfeeding, nonsteroidal anti-inflammatory drugs (NSAIDs), oral contraceptives (OCPs), and vitamin D levels. The data on the association of breastfeeding and UC are inconclusive, with some published studies suggesting a protective effect [33, 40], and other studies showing no significant effect [32, 41, 42]. Some published reports suggest that NSAIDs may precede the onset of IBD, lead to a reactivation of quiescent IBD, or exacerbate already active IBD in humans [43–46]. Among past and current smokers, OCP use was associated with a hazard ratio (HR) of 1.63 (95 % CI 1.13–2.35) for UC compared to non-OCP users [47] .
There has been increasing interest in the potential immunomodulatory role of vitamin D in the pathogenesis of diseases such as IBD [48]. In a large prospective cohort study of women, higher predicted 25-hydroxy vitamin D (25(OH)D) levels were associated with a significant reduction in the risk of incident CD, but only a nonsignificant decrease in the risk of UC.
Given the constant intestinal exposure to numerous luminal dietary antigens and the influence of dietary intake on the intestinal microbiota, investigators have long hypothesized a relationship between diet and UC [49, 50] . In a systematic review of case-control and cohort-based, nested case-control studies, authors examined macronutrients (fat, protein, carbohydrates) and specific foods (fruits, vegetables, meats), and the risk of IBD [51]. Increased dietary intake of total fats, polyunsaturated fatty acids (PUFAs) and meat were associated with an elevated risk of CD and UC. In contrast, high fiber and fruit intake were associated with a reduced risk of CD, whereas high vegetable intake was associated with reduced UC risk [51]. The sometimes conflicting results of multiple studies may reflect not only the potential methodological differences between studies, but also possibly the complexity of IBD pathogenesis itself [52]. Additional studies are needed to further define the potential role of diet in the pathogenesis of UC.
Immunology and Cell Biology
Activated cells of the mucosal immune system and elevated levels of pro-inflammatory cytokines and chemokines are present in the bowel of both UC and CD patients [18]. Previous studies implicate a dysregulation of activated intestinal ( cluster of differentiation, CD4 + ) T cell subtypes of the adaptive immune system in the pathogenesis of IBD. More recent studies however suggest CD and UC both may involve Th17 T cells, which rely upon the IL-23 pathway [18]. IL-23, an essential cytokine involved in the crosstalk between innate and adaptive immunity [19], is secreted by macrophages and dendritic cells and promotes expansion of Th17 cells [19, 53]. Elevated levels of IL-23 and Th17 cytokines are detected in the colonic mucosa of UC and CD [18, 54, 55].
In more recent years, genetic studies such as GWA studies have revealed the importance of the epithelial barrier integrity in IBD pathogenesis [19]. GWA studies have discovered associations between UC and susceptibility single nucleotide polymorphisms in the HNF4A, CDH1, LAMB1, and GNA12 regions, important in epithelial barrier function [56, 57]. These findings suggest that a disruption in the integrity of the epithelial barrier may play a role in the pathogenesis of UC. Linkage and candidate gene studies have determined variants in the XBP1 gene, associated with the unfolded protein response for both UC and CD [57–61]. Closely linked to autophagy and innate immunity, the unfolded protein response is triggered by endoplasmic reticulum stress due to accumulation of misfolded or unfolded proteins and is associated with Paneth cell function [57].
Clinical Signs and Symptoms
The typical symptoms of UC include rectal bleeding, diarrhea, and abdominal pain. The presentation can vary depending upon the extent of colonic involvement and severity of inflammation . The colon in UC is inflamed in a diffuse continuous distribution, extending from the rectum proximally. By convention, UC is classified according to the extent of disease into the following three subgroups: proctitis (disease limited to the rectum), left-sided colitis (disease extending to the sigmoid or descending colon, but not past the splenic flexure), and pancolitis (disease extending past the splenic flexure). The Paris classification system is a more detailed system developed for pediatric IBD clinical studies and includes detailed categories for UC disease location and severity, age, and growth status [62, 63]. Proctitis may present with tenesmus, urgency, and the passage of formed or semiformed stool with blood and mucus [64]. In contrast, pancolitis or left-sided disease may present with bloody diarrhea and significant abdominal pain. The majority of patients will present with a several week history of symptoms; however, some will present with a more acute clinical picture.
Although adults and children with UC can present with similar symptoms, there are differences in the clinical presentation of these two populations. Studies suggest that children with UC present with more extensive colonic involvement than adults with UC [3, 4, 65]. In a Danish study of 80 patients less than 15 years and 1080 patients 15 years of age or greater with UC, the younger group had more extensive disease at diagnosis compared to the older group diagnosed with UC [3]. Of the UC patients younger than 15 years of age, 29 % had pancolitis and 25 % had proctitis; in contrast, of the UC patients 15 years or older, 16 % had pancolitis and 46 % had proctitis [3] . Gryboski examined 38 children diagnosed with UC at ≤ 10 years old and reported 71 % with pancolitis, 13 % with left-sided colitis, and 6 % with proctitis [4]. Approximately 5 % of the children with UC have evidence of delayed linear growth and/or weight loss at diagnosis, although growth failure is much less frequent than in children with CD [4, 66] (see Table 30.2).
Clinical symptoms | Range of percentages |
|---|---|
Rectal bleeding | 75–98 |
Diarrhea | 71–91 |
Abdominal pain | 44–92 |
Weight loss | 13–74 |
Arthralgia/arthritis | 5–9 |
Fever | 3–34 |
Growth retardation | 4–5 |
Children with UC may present with varying degrees of disease severity. Approximately 50 % of the children with UC will present with a mild form of disease, characterized by an insidious onset of diarrhea and rectal bleeding, without abdominal pain or systemic symptoms such as fever. In these patients, disease may be confined to the distal colon [70, 71] . Disease of moderate severity is seen in 30 % of children with UC and is characterized by a more acute presentation with bloody diarrhea, tenesmus, and urgency; systemic symptoms including low-grade fever, abdominal tenderness, weight loss, and mild anemia may be present [70, 71]. Approximately 10 % of the children will present with a severe form of UC [70, 71]. Characteristic findings in severe disease include six or more bloody stools per day, fever, weight loss, anemia, hypoalbuminemia, and diffuse abdominal tenderness on physical exam [70–72]. A very small percentage of children will present initially with extraintestinal symptoms or manifestations, without obvious intestinal symptoms [71]. These extraintestinal manifestations of IBD may include axial or peripheral arthritis, erythema nodosum, pyoderma gangrenosum, or primary sclerosing cholangitis (see Sect. “Extraintestinal Manifestations”).
Diagnosis
The diagnosis of UC is established by the information gathered from a detailed symptom and family history, physical examination, and a combination of laboratory, radiologic, endoscopic, and histologic findings . It is important to exclude other etiologies such as an infectious process and to distinguish UC from CD. Colonic inflammation is typically characterized by bloody diarrhea with abdominal cramping. The differential diagnosis of colitis depends upon the age of the child at the time of evaluation. In infancy, necrotizing enterocolitis, Hirschsprung’s enterocolitis, and allergic colitis are diagnoses that should be considered. In children under age 3 who present with colitis, immunodeficiencies-causing colitis (such as IL-10 receptor deficiency and chronic granulomatous disease) should be excluded [73]. In contrast, in the older child and adolescent, enteric infection and idiopathic IBD are the most common diagnoses. (Causes of colitis are listed in Table 30.3.) In patients with painless rectal bleeding, other conditions (Meckel’s diverticulum, polyp) should be considered. In addition to details of the clinical presentation, the history should include family history, recent antibiotic therapy , infectious exposures, growth and sexual development, and the presence of extraintestinal manifestations of UC .
Table 30.3
Differential diagnosis of colitis. (Reprinted with permission from [67], Table 25.3, p. 389)
2. Infectious etiologies |
Campylobacter |
Salmonella |
Shigella |
E. coli 0157: H7 and other enterohemorrhagic E. coli |
Clostridium difficile |
Aeromonas |
Plesiomonas |
Entamoeba histolytica |
Cytomegalovirus |
Herpes simplex virus |
Yersinia |
Tuberculosis |
HIV and HIV-related opportunistic infections |
3. Other |
Ulcerative colitis |
Crohn’s disease |
Henoch–Schonlein purpura |
Hemolytic uremic syndrome |
Intestinal ischemia |
Intussusception |
Allergic colitis (primarily in infancy) |
Hirschsprung’s enterocolitis (primarily in infancy) |
Colitis complicating immunodeficiency |
Physical examination should include assessment of height, weight, and body mass index; abdominal distension, tenderness, or mass; extraintestinal manifestations (e.g., aphthous stomatitis, pyoderma gangrenosum, uveitis, or arthritis); fecal blood on rectal exam, perianal abnormalities (e.g., fistulae, fissures, or tags). Findings on physical examination may help to distinguish UC from CD; for example, pronounced growth failure or a perianal abscess strongly suggests the diagnosis of CD. A severely ill child with UC may have tachycardia, orthostatic hypotension, fever, or dehydration. Such findings in the presence of abdominal distention and a concerning abdominal exam may herald a fulminant presentation of UC with increased risk of developing toxic megacolon.
Laboratory Assessment
Initial laboratory evaluation should include appropriate blood tests, stool for occult blood, C. difficile toxin assay, and stool cultures. A complete blood cell count with differential may reveal a leukocytosis with or without left shift, anemia, and thrombocytosis. Thrombocytosis, hypoalbuminemia , and elevated erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) may indicate increased disease activity [74–76]. The presence of anemia with low mean corpuscular volume (MCV), wide red cell distribution width (RDW) and low iron levels may indicate an iron-deficient anemia secondary to ongoing fecal blood losses or the anemia of chronic disease. Children with UC may also have normal blood test results at the time of diagnosis [77] .
Fecal calprotectin and lactoferrin are frequently elevated in active UC [78, 79], and may be useful screening tests. Fecal biomarkers are elevated in patients with active UC and CD, but are not specific for IBD [80]. Fecal biomarkers cannot distinguish between different etiologies of mucosal inflammation and are increased in other conditions, including enteric infections [80]. Thus, enteric infections should be ruled out. Fecal calprotectin and lactoferrin are the most commonly studied fecal biomarkers. In a meta-analysis of six adult and seven pediatric studies, among children and adolescents with suspected IBD, the pooled sensitivity and specificity of fecal calprotectin testing were 0.92 (95 % CI, 0.84–0.96) and 0.76 (95 % CI, 0.62–0.86), respectively [81] .
It has been proposed that certain serum antibodies may be helpful for screening for IBD and discriminating UC from CD [82, 83]. Perinuclear antineutrophil cytoplasmic antibodies (p-ANCA) are seen in 60–80 % of adults with UC compared to 10–27 % of adults with CD [82, 84, 85]. Similarly, anti-Saccharomyces cerevisiae (ASCA) antibodies are commonly found in individuals with CD but are rarely seen in UC. In a study of 173 children, ASCA yielded a sensitivity of 55 % and specificity of 95 % for CD and ANCA had a sensitivity of 57 % and specificity of 92 % for UC [82]. In a study of 128 pediatric patients undergoing evaluation for IBD, Dubinsky and colleagues utilized modified cutoff values to optimize the sensitivity of the ASCA and ANCA assays. For the combination of ASCA and p-ANCA, the sensitivity of detecting IBD increased to 81 % with the modified values compared to the 69 % with standard cutoff values; however, this was accompanied by an increase in false positive rates among the children without IBD [83]. An overlap of ASCA and p-ANCA positive serology between patients with CD or UC remains. In particular, p-ANCA tends to test positive in the serum of patients with CD who exhibit UC features [82, 86]. Additional serological markers include antibodies to Escherichia coli outer membrane pori (OmpC), Pseudomonas fluorescens-associated sequence (I2), and flagellin (CBir1) [87]. In a study of greater than 300 children with suspected IBD, a serologic testing panel (including ASCA, p-ANCA, anti-OmpC and anti-CBir1) detected IBD with a sensitivity of 67 % and specificity of 76 %, compared to a sensitivity of 72 % and specificity of 94 % for a combination of three abnormal routine blood tests (hemoglobin, platelet count, and ESR) [87]. The value of these tests to supplement the routine diagnostic tests in IBD is a subject under study .
Endoscopic and Radiographic Evaluation
Evaluation with colonoscopy and ileoscopy with biopsies is the principal diagnostic test for UC. In patients with severe colitis, a limited flexible sigmoidoscopy examination with minimal air insufflation may be more appropriate, to avoid the risks of a full colonoscopy (perforation, hemorrhage, toxic dilatation). Upper endoscopy is also recommended by the “Porto” IBD Working Group of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN). Small-bowel imaging should also be performed to exclude small-bowel involvement, which may change the diagnosis from UC to CD. Options for small-bowel evaluation include upper GI with small bowel follow through, computed tomography (CT) scan , or magnetic resonance imaging (MRI) . The Porto expert panel has recommended MRI, over the other two modalities because of the absence of radiation; however, the quality of MRI varies from center to center, and small-bowel motion artifact may be sometimes confused with inflammation [88] .
In order to establish the extent of disease involvement by colonoscopy, we recommend biopsies from the terminal ileum and each segment of the colon, even if there are no visible findings at a particular level of the colon. In UC, typical findings seen by the endoscopist include a diffuse, continuous process starting at the rectum and extending more proximally into the colon. However, atypical features, such as rectal sparing, have been reported in children with UC [89–92]. In addition, UC patients with distal colitis can present with a “cecal patch,” discontinuous periappendiceal and cecal inflammation [92, 93]. The colonic mucosa often appears edematous, erythematous, and friable, with minute surface erosions and ulcerations (see Fig. 30.1). Larger, deeper ulcerations with associated exudate may develop in more severe disease. With long-standing UC, pseudopolyps may be present (see Fig. 30.2) [67]. In contrast, in CD, colonoscopy may reveal focal ulcerations (aphthous lesions) with intervening areas of normal-appearing mucosa (skip lesions). In severe or chronic CD, linear ulcerations, nodularity (cobblestoning), and strictures or stenoses may be present. In general, the ulcerations in CD are deeper and focal versus the diffuse, superficial ulcerations typical of UC (see Table 30.4) [89, 90, 94–97] .
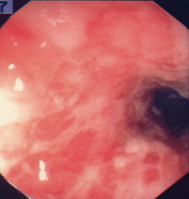
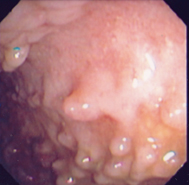

Fig. 30.1
Severe ulcerative colitis in a patient unresponsive to corticosteroid therapy. The colonoscopy demonstrates a featureless colon with loss of vascular pattern, ulcerations, and hemorrhage

Fig. 30.2
Multiple colonic pseudopolyps in a 17-year-old female with ulcerative colitis, with intermittent disease activity despite 2 years of maintenance therapy with thiopurine . (Reprinted with permission from [67], Fig. 25.2, p. 391)
Characteristic | Ulcerative colitis | Crohn’s disease |
|---|---|---|
Endoscopy findings | Diffuse continuous involvement extending from rectum | Focal lesions/disease interspersed with normal appearing mucosa (skip lesions) |
Rectum usually involveda | Rectal sparing possible | |
Diffuse, superficial, minute ulcerations; deeper ulcerations in severe disease | Aphthous lesions often surrounded by normal appearing mucosa; deep “collar button” ulcers; linear or serpiginous ulcerations | |
Strictures very rare | Strictures, often occurring in terminal ileum | |
Pseudopolyps | ||
Histopathology findings | No granulomasb | Granulomas (36 %) |
Diffuse chronic inflammation limited to the mucosac, crypt abscesses | Focal chronic inflammation, transmural inflammation | |
+ /− architectural distortiond |
Although by definition, the disease of UC is confined to the colon, children with CD or UC can have inflammation of the upper gastrointestinal tract. In a pediatric IBD registry study of 898 patients (643 with UC, 255 with CD colitis), authors examined the presence of macroscopic upper gastrointestinal involvement (ulcerations, aphthous lesions, or erosions) in accordance with the Paris classification system among the 260 patients with UC and 86 patients with CD colitis who underwent upper endoscopy with detailed documentation of lesions [62, 92]. Among the UC patients, gastric erosions were identified in 3.1 %, gastric ulcerations in 0.4 %, and erosions or ulcerations limited to the esophagus or duodenum in 0.8 %. In contrast, upper gastrointestinal lesions were noted in 22 % of the patients with Crohn’s colitis [92]. Except for the presence of granulomas of CD, it may be difficult to distinguish between UC and CD by the appearance of upper gastrointestinal lesions.
Plain abdominal radiographs and abdominal/pelvic CT scans evaluations may aid in the assessment for complications of UC, including toxic megacolon, perforation, or stricture. Plain abdominal radiograph may demonstrate thumbprinting, loss of haustral patterns, colonic dilatation (i.e., toxic megacolon), obstruction, or pneumoperitoneum (i.e., perforation of the bowel) [99–101] .
Pathology of Ulcerative Colitis
In active UC, typical findings on histopathology include a diffuse inflammatory cell infiltrate of the lamina propria mostly with plasma cells, lymphocytes, and neutrophils, but mast cells and eosinophils are also seen . Neutrophils invade the epithelium of the crypts, leading to cryptitis, crypt abscess formation, and goblet cell mucin depletion (see Fig. 30.3) [67]. The inflammatory infiltrate is typically confined to the mucosa, but in severe UC, ulceration may extend into the submucosa and deeper layers [96]. In quiescent (inactive) UC, the inflammatory infiltrate may diminish but signs of chronic colitis (architectural distortion, crypt branching and shortening, reduction in the number of crypts, and separation of crypts) can persist [96]. In children with UC, signs of chronic colitis (e.g., architectural distortion) are not always seen [91, 97].
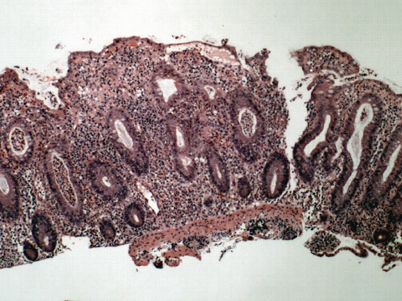

Fig. 30.3
Low power view of a colonic biopsy from a patient with active ulcerative colitis. Note the increased lamina propria inflammatory infiltrate, crypt abscesses, and crypt architectural distortion. The crypts are irregular in shape and placement and do not descend to the level of the muscularis mucosae. (Courtesy of Jonathan Glickman, MD, PhD, Director, Gastrointestinal Pathology, Miraca Life Sciences and Associate Clinical Professor of Pathology, Harvard Medical School). (Reprinted with permission from [67], Fig. 25.2, p. 393)
Histologic differentiation of UC from CD can be difficult. The histologic hallmark of CD is the noncaseating granuloma, which may be found in 25–48 % of children with CD [98, 102, 103]. However, a giant cell reaction mimicking a granuloma can occur around damaged crypts and spilled mucin. These “mucin granulomas” must be distinguished from true granulomas, which by definition, are not seen in UC [96]. Histologic skip areas, rectal sparing, focal inflammation, and transmural inflammation also suggest the diagnosis of CD. However, in children, histological skip areas may occur both at initial presentation of UC and as a result of therapy [89, 91, 104]. Rarely, children with UC present with focal active colitis at initial presentation [91]. In addition, patients with UC can have histological evidence of upper gastrointestinal inflammation similar to that in CD [105–107]. Given the overlap of histopathology findings in UC and CD, it may be difficult to distinguish between these two diagnoses if granulomas are not present .
If a clinician cannot reliably distinguish between CD and UC based on the available clinical, radiographic, and endoscopic data, an interim diagnosis of IBD-unclassified (indeterminate colitis) may be given until the patient can be more clearly classified in the future. The ESPGHAN Porto group developed detailed criteria to better define the IBD-unclassified subtype [88]. The prevalence of IBD-unclassified in adults and children with IBD is estimated to be 10–20 % [65, 108]. Approximately one third of these patients will later be classified as UC or CD [109] .
Extraintestinal Manifestations
Approximately 25–30 % of the children and adolescents diagnosed with IBD develop extraintestinal symptoms [110–113]. Extraintestinal manifestations of IBD may occur before, during, or after the development of gastrointestinal symptoms and may appear after surgical removal of diseased bowel [110, 114–117] . The clinical activity of the extraintestinal manifestations may or may not correlate with the activity of intestinal inflammation.
Joint manifestations (arthropathy) occur in 5–20 % of the children with UC [4, 112, 114]. These can be classified into two main clinical forms: a peripheral arthropathy and an axial arthropathy (e.g., ankylosing spondylitis) [110, 114]. Patients with IBD develop peripheral arthropathies in approximately 5–20 % of the cases [110, 111, 114, 118]. The peripheral arthropathy generally is asymmetrical, nondeforming and migratory, affecting mostly the large joints of the lower extremities including the knees, ankles, and hips. Less commonly the upper limb joints or hands are affected [110, 114, 118]. Small joints of the hands and feet generally are spared [114] .
Exacerbations of peripheral joint disease seem to parallelly increase the activity of bowel disease in UC or CD. The pauciarticular arthropathy is more likely to be correlated with exacerbations of bowel disease [117].
Axial arthropathies associated with HLA B27 occur in 1–4 % of patients [110, 111, 114, 118]. Ankylosing spondylitis associated with IBD runs a course independent of the activity of bowel disease, and may progress to permanent deformity [110, 114, 118]. In addition to the two main forms of joint manifestations, individuals with UC can develop isolated arthritis involving large joints including the sacroiliac joints, hips, and shoulders [118].
Pyoderma gangrenosum (PG) and erythema nodosum (EN) are the two main skin manifestations associated with UC and CD. PG occurs in < 1–5 % of the patients with UC [111, 115] and often is associated with active disease and extensive colonic involvement [115, 119] . The classic PG lesion often begins as a discrete pustule with surrounding erythema, then extends peripherally to develop into an ulceration with a well-defined border and a deep erythematous to violaceous color [120]. The lesions of PG tend to be multiple and localize below the knees, and can develop at sites of trauma and previous surgical sites, including scars and ileostomy stomas [119–121]. Approximately 40 % of the patients with UC and PG also develop joint symptoms [69]. EN occurs more frequently with CD (27 %) in comparison to UC (4 %) [111] and usually coincides with increased bowel disease activity [69, 111, 118]. EN lesions appear as tender, warm, red nodules, or raised plaques and usually localize to the extensor surfaces of the lower extremities [122]. Both PG and EN can precede the development of bowel symptoms and PG can occur after bowel resection [69]. Other skin manifestations include Sweet’s syndrome (acute febrile neutrophilic dermatosis) and oral lesions include aphthous lesions and pyostomatitis (pyoderma) vegetans [123] .
Ophthalmologic abnormalities are described in approximately 1–3 % of the children with IBD [110]. Uveitis and episcleritis are the more common ocular disorders reported [110, 124]. Uveitis associated with IBD in children may be asymptomatic, and thus, the incidence of associated eye findings may be underreported in the literature [125]. Ocular inflammation appears to develop more commonly in patients with other extraintestinal manifestations [125], including arthritis and may be associated with genes in the human leukocyte antigen (HLA) region [126]. In addition, corticosteroid use increases the risk of increased intraocular pressure and the development of posterior subcapsular cataracts [127, 128]. Given the potential eye complications, children with IBD should be monitored carefully at regular intervals .
Hepatic abnormalities in children with UC have been well described. While these are typically identified after the UC diagnosis, they may also precede the gastrointestinal symptoms [116, 129]. Transient elevations of alanine aminotransferase (ALT) occur in 12 % of the children with UC and appear to be related to medications or disease activity [129]. Persistent ALT elevations suggest the presence of primary sclerosing cholangitis (PSC) or autoimmune chronic hepatitis [129]. Among children with UC, approximately 3 % develop sclerosing cholangitis and < 1 % develop chronic hepatitis [129, 130].
The diagnosis of PSC may be established through a combination of cholangiography and liver biopsy [131]. There is a paucity of the literature addressing the long-term outcome of children with PSC specifically associated with UC [129]. In children with PSC (with or without IBD), later age at presentation, splenomegaly, and prolonged prothrombin time at presentation were associated with poor outcome, defined as death or listing for transplantation [116]. Patients with UC and PSC are also at increased risk for developing colon cancer. Fatty changes of the liver observed on liver biopsies of patients with UC or CD may be secondary to malnutrition, protein losses, anemia, and corticosteroid use [132] .
The IBD population has a threefold overall increased risk for venous thromboembolism (VTE) compared with individuals without IBD [133–135]. VTE occurs in 1–2 % of the children and adolescents hospitalized for active IBD [136, 137]. Although the incidence of VTE appears less than the rate noted in the adult population, these events can result in significant morbidity for younger patients [135]. Thrombotic events range from clots associated with venous catheters to more significant events such as deep vein thrombosis, pulmonary embolism, and cerebral vascular accidents. These events can lead to ongoing associated complications and long-term sequelae, including permanent neurologic deficits, persistent or recurrent thrombosis, embolization, and postthrombotic syndrome [136]. Factors that can further increase the risk for thromboembolism include an indwelling central venous catheter , severe disease activity, older age, parenteral nutrition, known thrombophilia, first-degree family history of VTE, persistence of anti-phospholipid antibody for greater than 12 weeks, oral contraceptives, smoking, obesity, and medications associated with increased risk of thrombosis such as thalidomide [136, 137] .
At a minimum, all hospitalized children and adolescents with IBD should maintain adequate hydration and receive mechanical thrombotic prophylaxis, such as encouraged ambulation, compression stockings, or pneumatic boots [137, 138]. The role of primary pharmacologic prophylaxis for thromboembolism remains controversial for children and adolescents with IBD. At our institution, we have implemented a risk stratification strategy to identify hospitalized IBD patients at higher risk for thrombosis, who warrant for primary prophylaxis pharmacologic anticoagulation therapy [136, 137]. For IBD patients who develop thromboembolism, anticoagulation with low-molecular-weight heparin appears feasible without major sequelae of increased bleeding or complications [136] .
Rarely, other hematologic abnormalities associated with UC occur, including immune thrombocytopenic purpura [139] and autoimmune hemolytic anemia [140]. Osteopenia–low bone mineral density occurs in children with UC, but less often than in children with CD [141, 142]. Corticosteroid use increases the risk of osteopenia in children with IBD [141, 142]. Other extraintestinal manifestations include nephrolithiasis [111, 143], pancreatitis (related or unrelated to medications) [144, 145], and pulmonary and cardiac involvement [110] .
Complications
Complications of UC include massive hemorrhage, toxic megacolon, perforation of the bowel, strictures, and colon cancer . Massive hemorrhage can occur with severe UC and is managed with blood transfusions and treatment of the underlying UC, and urgent colectomy may be required. One consensus group suggested that an individual with UC who requires more than 6–8 units of blood in the first 48 h and is still actively bleeding should undergo a colectomy [146]. Colonic perforation is the most dangerous complication of UC, can occur in the setting of severe UC with or without toxic colonic dilatation [147–149], and requires urgent colectomy.
Toxic megacolon is a potentially life-threatening complication of UC and is characterized by total or segmental nonobstructive colonic dilatation of at least 6 cm in adults associated with systemic toxicity [148, 150, 151]. Previous reports suggest a lifetime risk of toxic megacolon complicating IBD of 1–5 %, but this has decreased more recently, probably secondary to earlier recognition and improved management of severe colitis [148, 152]. Most likely, the pathogenesis of toxic megacolon is multifactorial [148]. In contrast to typical UC, in which the inflammatory changes are limited to the mucosa, in toxic megacolon, the severe inflammation extends into the deeper layers of the colonic wall [147]. It is thought that the spread of the inflammatory process to the smooth-muscle layer may lead to the paralysis of the colonic smooth muscle and subsequent dilatation of the colon [148] .
Several triggering factors have been reported to precede the development of toxic megacolon [147, 148]. Medications that can impair colonic motility should be avoided and have been implicated as precipitating factors, including narcotic agents for pain or antidiarrheal effects, anticholinergic agents, drugs that decrease motility, or antidepressants with significant anticholinergic effects [147, 153, 154]. A barium enema or colonoscopy may cause distention that can further impair the colonic wall blood supply and may increase the mucosal uptake of bacterial products [148]. Barium enema examinations have been reported in proximity to the development of toxic megacolon [154, 155]. The early discontinuation or rapid tapering of steroids or 5-aminosalicylic acid agents may contribute to the development of toxic megacolon [147, 148] . Electrolyte abnormalities, such as hypokalemia, have been observed in the setting of toxic megacolon, though it is not clear whether this finding is a causative factor or secondary to the illness itself [147]. Along with colonic dilatation, patients with toxic megacolon present with systemic findings including fever, tachycardia, leukocytosis, and anemia [150]. A decrease in the number of stools may herald the onset of toxic megacolon. With progressive disease, these individuals can develop dehydration, mental status changes, electrolyte disturbances, hypotension, and increasing abdominal distension and tenderness, with or without signs of peritonitis [148, 150] .
Abdominal X-ray reveals colonic dilatation, most frequently involving the transverse colon, sometimes accompanied by inflammatory changes including absent or markedly edematous haustral pattern [156] (see Fig. 30.4). Because the transverse colon is the most anterior portion of the colon, air will tend to accumulate in this segment of the colon when the patient is in the supine position; however, with repositioning of the patient, the colonic air will redistribute, filling other segments of the bowel [157].
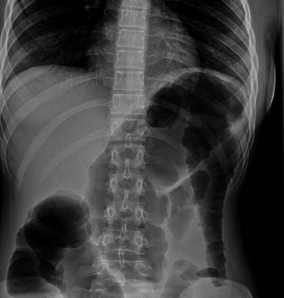

Fig. 30.4
Toxic megacolon in a teenager with fulminant ulcerative colitis. There is a massively dilated loop of transverse colon. This patient developed the megacolon despite corticosteroid therapy, and subsequently underwent emergent surgery. (Courtesy of Anne Wolf, M.D. and Matthew Egberg, M.D., Division of Gastroenterology, Hepatology and Nutrition, Boston Children’s Hospital)
The management of toxic megacolon is detailed in multiple reviews elsewhere [147, 148]. If toxic megacolon is present, surgical consultation is essential and the patient will most likely require a colectomy. Early surgical intervention is indicated in the setting of failed medical therapy with progressive colonic dilatation, worsening systemic toxicity, perforation, or uncontrolled hemorrhage [148] .
Both benign and malignant colonic strictures can develop in long-standing UC [158–160]. Benign strictures present most commonly in the rectum and the sigmoid, are due to smooth muscle hypertrophy, and are thought to be potentially reversible [158]. Colonic strictures should be evaluated for possible malignancy, but the majority of strictures in UC are benign [158–160]. There is an increased risk of dysplasia and colon cancer in patients with long-standing UC, which is addressed later in this chapter (see Sect. “Prognosis/Follow-Up”).
Treatment Options
The treatment goals for children with UC are the control of active disease and induction of remission, the long-term maintenance of remission, and provision of education and psychosocial support for the patient and family. The initial treatment of UC is medical, with surgery reserved for patients with severe disease, patients with medically refractory disease, or patients who develop adverse effects of medical therapy .
Knowledge of the extent and severity of disease involvement will enable the clinician to choose the appropriate therapy for each individual patient. Distal UC (left-sided UC or proctitis) is characterized by involvement limited to the area distal to the splenic flexure, and potentially may be treated with topical agents (e.g., aminosalicylate or hydrocortisone enemas or suppositories). Extensive UC is defined by involvement extending proximal to the splenic flexure and requires systemic therapies with or without additional topical agents. Severity of disease is usually simple to ascertain, and can be determined by assessing stool frequency and consistency, abdominal pain, nocturnal diarrhea, hematocrit, albumin level, and feeding intolerance. Separate published disease severity criteria for adults and children have been developed by Truelove and Witts, and Werlin and Grand, respectively [72, 161]. Typically, severe colitis requires hospitalization and administration of either intravenous corticosteroids or other immunosuppressive agents (e.g., cyclosporine, tacrolimus, infliximab) . Many patients with severe colitis will require colectomy .
Turner and colleagues developed the pediatric ulcerative colitis activity index (PUCAI), a noninvasive, clinically based, validated instrument to assess disease activity in pediatric patients with UC [162]. The PUCAI is a score comprised of six parameters, including the assessment of abdominal pain, rectal bleeding, stool consistency, number of stools per 24 h, nocturnal stools, and activity level. Each item is assigned a value contributing to a combined, total PUCAI score ranging from 0 to 85. Categories of UC disease activity are defined by the following total PUCAI scores: 0–9 (no activity), 10–34 (mild activity), 35–64 (moderate activity), and 65–85 (severe activity). The PUCAI correlated well with the physician’s global assessment, Mayo score, and macroscopic findings at colonoscopy. In addition to excellent interobserver and test–retest reliability, the PUCAI performed well on longitudinal assessment with excellent responsiveness at repeated visits, thus providing a tool to serially assess patients’ clinical status [162]. Studies demonstrate the application and feasibility of the PUCAI in hospitalized pediatric patients with severe colitis and in the outpatient setting [163, 164]. Although the PUCAI provides a method to track UC disease activity, it does not replace the need for the appropriate serial thorough clinical evaluation of children and adolescents with active UC. Presently, there is no pediatric-specific UC endoscopic index available .
Induction Therapy
Mild to Moderate Colitis
In the child with mild to moderate UC, with no or only minimal systemic signs (such as elevated ESR or mild anemia), aminosalicylates (e.g., sulfasalazine, olsalazine, mesalamine, balsalazide) are usually the first line of therapy (see Tables 30.5 and 30.6).
Oral preparations | Dosage form | Mechanism of release | Site of delivery |
|---|---|---|---|
Azo-bond | |||
Sulfasalazine (Azulfidine) | 500 mg tablet | Bacterial cleavage of azo bond | Colon |
Olsalazine (Dipentum) | 250 mg capsule | Bacterial cleavage of azo bond | Colon |
Balsalazide (Colazal/Colazide) | 750 mg capsule | Bacterial cleavage of azo bond | Colon |
Delayed release | |||
Mesalamine (Delzicol/Asacol HD) | 400 mg/800 mg tablets | pH-dependent breakdown (pH > 7) | Distal ileum to colon |
Mesalamine (Salofalk/Mesasal/Claversal) | 250 mg/500 mg tablets | pH-dependent breakdown (pH > 6) | Ileum to colon |
Extended, delayed release | |||
Mesalamine | |||
(Lialda) | 1.2 g tablet | pH-dependent breakdown (pH > 7) | |
(Apriso) | 375 mg capsule | pH-dependent breakdown (pH > 6) | |
Sustained release | |||
Mesalamine (Pentasa) | 250 mg/500 mg/1000 mg tablets | Ethylcellulose-controlled time-release | Small intestine to colon |
Rectal preparations | |||
Mesalamine suppository (Canasa–500 mg) | 400 mg/500 mg/1000 mg | Rectum | |
Mesalamine enema (Rowasa–4 g/60 ml) | 1 g/4 g; 60-ml/100-ml suspension | Rectum to splenic flexure | |
Medication | Dosage | Major side effects |
|---|---|---|
Sulfasalazine | 50–75 mg/kg/day PO divided bid or tid | Nausea, headaches, diarrhea, photosensitivity, hypersensitivity reaction, pancreatitis, azoospermia, hemolytic anemia, neutropenia |
(Maximum 6 g/day) | ||
Adult dose: 3–4 g/day divided bid or tid | ||
Mesalamine | ||
Oral formulation | 50–75 mg/kg/day PO divided qid, tid, or bid (may vary according to preparation) | Nausea, headaches, diarrhea, pancreatitis, nephritis, pericarditis, pleuritis |
(Maximum 6 g/day) | ||
Adult dose: 3–4 g/ day divided qid, tid, or bid | ||
Enema formulation | 2–4 g PR q 12–24 h | |
Suppository formulation | 500 mg PR q 12–24 h | |
Corticosteroids | ||
IV or oral formulation | 1–2 mg/kg/day of prednisone or equivalent, IV or PO, divided q 12–24 h (maximum 60 mg/ day) | Numerous, including Cushing’s syndrome, growth suppression, immunosuppression, hypertension, hyperglycemia, increased appetite, osteoporosis, aseptic necrosis (hip), cataracts |
Enema formulation | 50–100 mg of hydrocortisone PR qhs | |
Suppository formulation | 25 mg of hydrocortisone acetate PR qhs | |
Azathioprinea | 1.5–2.5 mg/kg/day PO qd | Nausea, emesis, immunosuppression, hepatotoxicity, pancreatitis, myelosuppresion, lymphoma |
6-Mercaptopurinea | 1.0–2.0 mg/kg/day PO qd | Nausea, emesis, immunosuppression, hepatotoxicity, pancreatitis, myelosuppresion, lymphoma |
Cyclosporine | Induction regimen for fulminant colitis: initial dose: 4 mg/kg/day IV continuous or bid; maintenance oral dose varies according to oral preparation | Nephrotoxicity, hypertension, headache, hirsutism, nausea, emesis, diarrhea, tremor, hypomagnesemia, hyperkalemia, hepatotoxicity, seizures, gingival hyperplasia, lymphoproliferative disorder |
Tacrolimus | 0.2 mg/kg/day PO divided bid | Nephrotoxicity, hypertension, headache, immunosuppression, nausea, emesis, tremor, hypomagnesemia, elevated liver enzymes, hyperglycemia, seizures, lymphoproliferative disorder |
Infliximab | Loading dose: 5 mg/kg/dose IV at 0, 2, 6 weeks; maintenance dose: 5 mg/kg/dose IV q 4–8 weeks (can go up to 10 mg/kg every 4 weeks) | Infusion reactions, delayed hypersensitivity reactions, immunosuppression (opportunistic infections), lupus-like syndrome, hepatotoxicity, blood dyscrasias, psoriasis, neurotoxicity (demyelination), vasculitis, possible lymphoma |
Adalimumab | Adult dosing—Loading dose: 160 mg, then 80 mg; maintenance dose: 40 mg SQ every other week (can go up to 40 mg weekly) | Injection site pain/ reactions, immunosuppression (opportunistic infections), psoriasis, blood dyscrasias, possible lymphoma |
Mesalamine is a 5-aminosalicylic acid compound used in the induction and maintenance treatment of UC. It was discovered as the active anti-inflammatory moiety of sulfasalazine, which has been used to treat UC since the 1940s [166]. More than 88 % of all the UC patients receive treatment with aminosalicylate (ASA) agents; however, fewer than 50 % of the children with UC can maintain long-term steroid free remission on ASA monotherapy [167]. Sulfasalazine contains mesalamine bound to sulfapyridine via an azo bond, which is released by bacterial azo-reductase in the small bowel and colon. Sulfapyridine is inactive, but is absorbed in the colon and is mostly responsible for hypersensitivity reaction and adverse effects associated with sulfasalazine [168].
ASA agents have multiple immunologic effects. Potential mechanisms of action include the inhibition of the synthesis of leukotriene B4, a potent chemotactic and chemokinetic agent, and the inhibition of the activation of nuclear transcription factor kappa B (NF-kappaB), an important mediator of the immune response in inflammatory processes [169–171].
Controlled studies suggest that currently available ASAs are superior to placebo for induction of remission and relapse prevention [172–175]. However, there do not appear to be any differences in efficacy between the older agent, sulfasalazine, and newer ASA drugs [175, 176].
Potential advantages of the non-sulfa ASA agents include better tolerance compared to sulfasalazine [176, 177] and the availability of a non-sulfa ASA agent for sulfa-sensitive individuals. In adult-onset UC, balsalazide at higher doses (6.75 g/day) may provide a faster improvement in active, mild-to-moderate UC than lower doses of balsalazide (2.25 g/day) or mesalamine (2.4 g/day) [178, 179].
Long-acting formulations of mesalamine are available, however there is no FDA approved pediatric dose. They are available as a delayed release multi-matrix formulation (Lialda) and a pH-controlled granule that releases mesalamine at pH > 6 (as in the colon, Apriso).
Common side effects of sulfasalazine include headache, nausea, and fatigue, which improve with reduction of the dose [180] (see Table 30.6). The sulfa moiety can cause hypersensitivity reactions resulting in rash, fever, hepatitis, hemolytic anemia, bone marrow suppression, and pneumonitis [180, 181]. Other side effects include neutropenia, oligospermia, pancreatitis, and the exacerbation of colitis [72, 182]. Folic acid supplementation is recommended given that sulfasalazine impairs the absorption of folic acid and may lead to anemia [180]. To decrease side effects, sulfasalazine is started at a dose of 10–20 mg/kg/day and gradually increased to the full dose (50–75 mg/kg/day) over 5–7 days mesalamine and the other non-sulfa ASA agents have also been associated with adverse reactions, including pancreatitis , hepatitis, nephritis, exacerbation of colitis, and pneumonitis [180, 183, 184].
Moderate Colitis
Children with moderate disease are usually managed with oral corticosteroids (usually 1 mg/kg/day, up to 60 mg/day of prednisone) as an outpatient. Corticosteroids are effective for short-term treatment, but up to 45 % of the pediatrics patients develop corticosteroid dependence in subsequent years putting them at risk for steroid-related side effects [185].
Potential short-term complications of steroid therapy in patients with UC include increased appetite, weight gain, fluid retention, mood swings, hyperglycemia, hypertension, insomnia, acne, and facial swelling. Complications of long-term steroid therapy (usually of greater than 3 months) include growth retardation, osteopenia with compression fractures, aseptic necrosis of the hip, and cataracts [180, 186]. Given these reasons and the suppression of the hypothalamic–pituitary–adrenal axis, corticosteroids should be tapered shortly after remission is achieved. A standard taper utilized by the authors is reduction by 5 mg/week of prednisone down to 20 mg/day, and then a more gradual taper on alternate days, aiming for 10 mg every other day with further taper and cessation if remission is maintained [186]. The high frequency of side effects with traditional systemic steroids such as prednisone has led to the development of steroid preparations with high first-pass metabolism and few systemic effects.
Severe Colitis
Children with severe disease typically defined by a PUCAI greater than 65 (e.g., more than five bowel movements/day, liquid bloody stools, or severe pain with defecation, anemia, and hypoalbuminemia) require intravenous corticosteroids (methylprednisolone at 40–60 mg/day, divided into two doses/day, approximately 1–2 mg/kg/day) and hospitalization for further evaluation, observation, and management [187, 188]. Rectal corticosteroids or 5-ASA agents may be used as an adjunctive therapy with parenteral corticosteroids for patients with severe tenesmus [180]. Intravenous fluid for rehydration and correction of electrolyte imbalances should be provided. Blood transfusions and albumin infusions may be required. Although traditionally patients are restricted from taking food orally, there is no data to support holding the oral diet in UC as there is in CD; however, if bowel rest is indicated, the child may require central venous access for parenteral nutrition support [189, 190].
In addition to high-dose steroids, empiric antibiotics are sometimes used in severe colitis, though the efficacy of antibiotics has not been proven [191–194]. Assessment for response to intensive medical therapy includes resolution of fever, tachycardia, abdominal tenderness, and macroscopic blood per rectum. Stools should be decreasing in frequency, but may still be unformed. Following the PUCAI will help guide therapy. Once the child shows significant improvement, diet is advanced to a low-residue diet, intravenous methylprednisolone is switched to oral prednisone, and similar parameters for steroid wean are followed as outlined above. The optimal duration of intravenous corticosteroid therapy is unclear, but most children will respond within 7–10 days.
Approximately 50 % of the patients treated with intravenous corticosteroids will not respond. Turner and colleagues have published an excellent consensus statement on the use of rescue agents in the management of severe UC. In summary, the PUCAI can be utilized to track disease activity. If the child has not responded to steroid therapy (as assessed by a PUCAI drop of 20 points or more after 5–7 days), the options of surgery or more intensive immunosuppression (intravenous cyclosporine, infliximab, or oral tacrolimus) should be considered and discussed with the family. Intensive immunosuppression should not be started if surgery is believed imminent, such as in a septic patient, a patient with toxic megacolon, or a patient with a suspected perforation. Flexible sigmoidoscopy is indicated prior to the institution of calcineurin inhibitors or biologics to exclude cytomegalovirus [195].
The medical agents utilized to treat steroid refractory colitis in the hospitalized patient are calcineurin inhibitors and biologics. In the past decade, infliximab has been the agent most commonly utilized, perhaps due to its ease of administration and more favorable side-effect profile. Approximately 70 % of the children and adults treated with infliximab will respond [164, 196]. The role of monitoring infliximab levels during treatment of severe colitis is still a topic under study. However, patients with severe colitis may have an increased loss of infliximab in the stool, and may require higher more frequent dosing than the 5 mg/kg utilized in the clinical trials (which included less sick patients) [197]. Adalimumab also has been utilized in severe colitis [198]. Calcineurin inhibitors such as cyclosporine and tacrolimus are reasonable alternatives to biologics, and have been utilized for over 20 years. However, because of long-term renal toxicity, it is generally recommended that these agents be utilized as short-term induction agents for a few months, and that patients should transition to maintenance therapy with thiopurines or anti-tumor necrosis factor-alpha (anti-TNF) agents [199]. Calcineurin inhibitors may also be utilized as a steroid sparing “bridge” to surgery [200]. The timing of colectomy in a child who is not responding to intravenous corticosteroids or other immunosuppression can be difficult. It should be emphasized that even if a child responds to immunosuppression, such as cyclosporine, there is a high likelihood that he/she will require a colectomy within a year [201, 202]. If immunosuppression with cyclosporine is used and the child does not respond to these medications within 10–14 days, surgery should be strongly recommended.
Close monitoring of the patients with severe colitis for the development of fulminant colitis and associated complications including hemorrhage, toxic megacolon, and perforation is essential using serial abdominal exams, complimented by serial abdominal films or other imaging as necessary. Physical examination should assess for evidence of worsening abdominal tenderness, distention, and hypoactive bowel sounds; these may herald the development of toxic megacolon. The appearance of persistent abdominal pain and distention, diffuse abdominal tenderness and rebound, fever and tachycardia are worrisome signs of an acute abdomen and may signal need for emergent surgical intervention. Opiates and loperimide should be avoided given the increased risk of developing toxic megacolon [153]. Steroid therapy may mask the typical symptoms and signs of perforation, but this may be detected by serial upright abdominal films (see Sect. “Complications”).
Left-Sided Colitis/Proctitis
Topical ASA or topical corticosteroids are effective in the treatment of proctitis, proctosigmoiditis, or left-sided UC [203–205] (see Tables 30.5 and 30.6 ). To be effective, topical therapy must reach the most proximal extent of the disease activity. Mesalamine enemas or suppositories are effective as first-line/maintenance therapy for mild or moderately active left-sided UC or proctitis, respectively [180]. Rectal mesalamine may be superior to oral mesalamine in the treatment of active ulcerative proctitis [206]. Mesalamine enemas may be superior to rectal corticosteroids [207] and are also effective in treating distal colitis that is unresponsive to oral ASAs or corticosteroids [208, 209]. Combination therapy with oral and topical mesalamine is more effective than one agent alone in the treatment of mild to moderate distal colitis [210]. Mesalamine suppositories spread to the upper rectum, and enemas and foams can reach the splenic flexure or into the distal transverse colon [208, 209].
Corticosteroid suppositories or enemas also can be used as first-line induction therapy in patients with mild or moderately active ulcerative proctitis or left-sided UC [180]. Rectal administration of hydrocortisone or prednisolone permits more direct delivery of steroids to distal UC sites; however, as with oral steroid therapy, prolonged treatment with topical steroids may induce systemic steroid side effects, including adrenal suppression [205, 209]. Topical agents such as budesonide enemas may induce remission in distal colitis with fewer systemic steroids side effects [211, 212]. Some evidence suggests that ASA enemas may be superior to hydrocortisone enemas [204, 207, 209].
Maintenance Therapy
5-Aminosalicylic Acid Agents
Medication for the prevention of relapse after induction of remission often is started before the induction therapy is discontinued. The clinician usually aims to transition a patient from corticosteroids onto sulfasalazine or another ASA agent. Multiple studies of adults with UC demonstrate the effectiveness of sulfasalazine and other ASA agents in preventing relapse [176]. Mesalamine is well-tolerated in the long-term treatment of children with IBD, with the principal adverse event being exacerbation of diarrhea [213]. Sulfasalazine and newer ASAs are all effective in maintaining remission in UC [177]. Balsalazide, at a higher dose (6 g/day), also may be more effective in preventing relapses of UC in adults, compared to lower-dose balsalazide (3 g/day) and mesalamine (1.5 g/day) [214]. Topical mesalamine can effectively prevent relapse of active distal UC [215], but because of the rectal route of administration, patients may prefer oral mesalamine for maintenance therapy. The combination of oral and topical ASA therapy may be more effective in preventing relapse than oral ASA therapy alone, especially for distal disease [216]. Children with UC require years of maintenance therapy. The exact duration that a UC patient in remission should remain on maintenance therapy is unclear, and there are no formal guidelines on when or if maintenance therapy should be discontinued. The risk of discontinuing maintenance medication is the possibility of relapse. In addition, maintenance with ASA or other agents may reduce the risk of colorectal neoplasia [217–219].
6-Mercaptopurine and Azathioprine
The immunomodulatory drugs, azathioprine (AZA) and its metabolite 6-mercaptopurine (6MP), can reduce disease activity and allow the withdrawal of steroid therapy in children with steroid-dependent UC [220, 221].
AZA is nonenzymatically metabolized to 6MP, and the two drugs have identical mechanisms of action. AZA is 55 % 6MP by molecular weight and therefore dosed higher mg per kg. As AZA is the prodrug of 6MP, the dose of AZA is approximately twice that of 6MP [222]. The mechanism of action of 6MP and AZA is to interrupt RNA and DNA synthesis, thereby downregulating cytokines, T cell activity, and delayed hypersensitivity reactions and by inducing T cell apoptosis by blocking the activation of the gene rac-1 [223].
The use of thiopurines in UC as steroid sparing maintenance agents is long established. Given their steroid-sparing effects [220, 221] and reasonable tolerance by children with IBD [224], AZA and 6MP offer an alternative maintenance treatment of IBD in children. A prospective multicenter registry study in pediatric UC patients found 50 % of children with UC starting thiopurine therapy were disease free 1 year later, without the need for rescue therapy [225].
The metabolism of AZA/6MP is well established. AZA is converted to 6MP, and then to 6-thiouric acid (6-TUA), 6-methyl mercaptopurine (6-MMP), and 6-thioguanine nucleotide (6-TGN) [226, 227]. Evaluation for thiopurine methyltransferase genetic polymorphism should be obtained prior to the institution of 6MP or AZA therapy as it can identify those children at higher risk for drug toxicity [228] and the starting dose of thiopurine can be adjusted accordingly. The 6-TGN moiety is thought to be the active component responsible for the inhibition of lymphocyte proliferation by DNA breakage. There is data demonstrating increased 6-TGN level correlating with increased response to therapy in IBD, with increased 6-TGN levels associated with clinical remission [229]. Dosage can be adjusted using metabolite levels. as the target 6-TGN level is found to be 235 pmol/8 × 108 red blood cells and 6-MMP level < 5700 pmol/8 × 108 red blood cells [230, 231]. The 6-MMP fraction is thought to be responsible for the hepatotoxic effects of AZA and 6MP [222, 229, 231, 232]. A 6-MMP level > 5700 pmol/8 × 108 red blood cells is found to lead to a threefold increase risk of hepatotoxicity [231].
Allopurinol is a xanthine oxidase inhibitor used to treat gout as its primary indication. In patients that shift the breakdown of 6MP and AZA preferentially towards an increased 6-MMP fraction, the addition of allopurinol allows a lower dose of AZA/6MP with increased 6-TGN levels and lower comparative 6-MMP fraction. There is limited published data for dosage in pediatric IBD patients [233].
An alternate approach to maximize 6MP/AZA effect in patients with elevated transaminases due to preferential metabolizers of thiopurines is split dose administration. The total daily dose is the same, but divided into two equivalent doses, and this is found to decrease transaminases as well as flu-like symptoms which can be seen with 6MP/AZA use. This has been demonstrated in a retrospective adult study, and has been used in the pediatric UC population [234].
Given the high relapse rate with withdrawal of 6MP in adults with UC [235], the majority of children requiring AZA or 6MP to suppress disease activity most likely will require long-term maintenance therapy with these agents. There are no good data in UC addressing the question of when (or if) immunomodulators should be discontinued after a patient has entered remission. Most patients are continued on the medication for several years if they respond to 6MP or AZA. There is an approximate fourfold increase risk of lymphoma in IBD patients treated with AZA or 6MP. It is unclear if this is due to the medications themselves or due to the severity of the underlying disease [236]. In addition, there is a slightly increased risk of Epstein–Barr virus associated lymphoma in a large cohort of patients treated with long-term 6MP or AZA [237].
Methotrexate
Methotrexate (MTX) is a dihydrofolate reductase inhibitor used for induction and maintenance in patients with CD [238]. At low dose, MTX’s mechanism of action is not clearly defined. At high doses, it works through antiproliferative and cytotoxic effects by inhibiting dihydrofolate reductase, leading to defective DNA synthesis and cell death [239]. At low doses, it works primarily as an immunomodulator [240]. The mechanism of action as an immunomodulator is not clearly understood but involves adenosine inhibition, induction of apoptosis, and decreased IL and eicosanoids to decrease inflammatory mediators [241–243].
There is some evidence from open label trials in adults to suggest the use of MTX monotherapy as maintenance in UC, but there is very little supportive data in the pediatric population. A multicenter randomized controlled trial of MTX use in adults with chronic active UC found weekly oral MTX at 12.5 mg to be no better than placebo in induction or maintenance of remission in patients with chronic active UC [244]. A small retrospective pediatric paper reported response or remission in 72, 63, and 50 % of patients at 3, 6, and 12 months, respectively [245]. Larger controlled prospective trials would be needed to show clear benefit for MTX monotherapy in pediatric UC patients [246].
Biologic Agents
Infliximab
Infliximab is a monoclonal antibody to tumor necrosis factor-alpha which is a cofactor in the production of inflammatory cytokines, gamma interferon, and IL-2 [247, 248]. The efficacy of infliximab in pediatric patients was demonstrated in “the REACH trial” which was a prospective trial demonstrating 73 % response at 8 weeks in pediatric UC patients with moderate to severely active UC using 5 mg/kg at 0, 2, and 6 weeks, followed by either every 8 week or every 12 week dosing interval. The response at 54 weeks in the every 8-week group was double that of the every 12-week maintenance group [249, 250].
Combination therapy with infliximab plus AZA has been shown to be more effective in an adult study [251] with better response of steroid free remission at 16 weeks in the combination therapy group. There are no similar studies in the pediatric patient population [251]. The potential risks of combination therapy including the possible development of lymphoma as previously discussed and the above data make the risk-versus-benefit evaluation of combination therapy critical.
Adalimumab
Adalimumab is a fully human monoclonal antibody that binds to tumor necrosis factor-alpha. It was approved for adults with UC in 2012. It is currently not approved for use in pediatric UC patients. The adult literature comparing adalimumab to placebo prospectively showed a superior response of clinical remission in treatment group at both week 8 (17 vs. 9 %) and week 52 (17 vs. 9 %), respectively. Anti-TNF naïve patients had double the response to therapy compared to placebo as well (21 vs. 9 % at week 8 and 22 % vs. 10 % at week 52, respectively) [252].
Vedolizumab
Vedolizumab is a humanized monoclonal antibody that specifically recognizes alpha-4 beta-7 integrin, a cell survive glycoprotein variably expressed on circulating B and T lymphocytes. This interacts with mucosal addressin-cell adhesion molecule-1 (MAdCAM-1) [253] on intestinal vasculature [254, 255]. Vedolizumab has been found more effective than placebo for induction and maintenance therapy for UC. In randomized placebo-controlled adult studies, induction response was 47 % compared with 25.5 % in placebo at 6 weeks, and maintenance response was achieved in 41.8 % in treatment group at every 8 weeks, 44.8 % at every 4 weeks at 300 mg compared with 15.9 % in placebo group. There was a similar frequency of adverse events in both treatment groups and placebo groups. This effect appears to be more effective in patients who have previously lost response to anti-TNF therapy [256].
Other Therapies
Antibiotics
Despite the potential role of infectious agents in the pathogenesis of UC [20], the use of antibiotic therapy in the treatment of UC remains controversial. There is a lack of consistent evidence in the effectiveness of antibiotics in the induction and maintenance of remission in UC [192, 257–260]. In one study, oral tobramycin therapy improved short-term clinical and histological outcomes, [257] but there was no advantage in the prevention of relapse compared to placebo [258]. In two studies of active UC, the addition of 10–14 days of oral or intravenous ciprofloxacin to corticosteroid therapy did not improve rates of remission [194, 261]. In another placebo-controlled study, 6 months of treatment with ciprofloxacin, in addition to standard therapy with prednisone and mesalamine, resulted in a greater clinical response compared to placebo; however, this advantage was not sustained after the cessation of ciprofloxacin [262]. Empiric broad-spectrum antibiotics often are administered in the setting of severe active UC [72, 191], especially if there is concern for potential fulminant colitis or toxic megacolon [148].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







