Autologous grafts:
Fascia lata
Rectus fascia
Allografts:
Human cadaveric dermis:
AlloDerm (LifeCell Corporation, Branchburg, New Jersey, USA)
FlexHD (Ethicon, Cornelia, Georgia, USA)
Cadaveric fascia lata:
Suspend Tutoplast (Mentor Corporation, Santa Barbara, California, USA)
FasLata (CR Bard, Covington, Georgia, USA)
Xenografts:
Porcine dermis:
Cross-linked:
Permacol (Covidien, Mansfield, Massachusetts, USA)
Collamend (Davol, Warwick, Rhode Island, USA)
Pelvicol Acellular Collagen Matrix (CR Bard)
PelviSoft BioMesh (like the above, only with perforations)
(CR Bard)
Non-cross-linked:
Strattice (LiefeCell Corporation)
XenMatrix (Davol)
Porcine small intestinal submucosa:
Surgisis (Cook Surgical, Bloomington, Indiana, USA)
Bovine dermis:
Xenform Soft-Tissue Repair Matrix (Boston Scientific, Natick, Massachusetts, USA)
SurgiMend (TEI Biosciences, Boston, Massachusetts, USA)
Bovine pericardium:
Veritas (Synovis Surgical Innovation, St Paul, Minnesota, USA)
Tutomesh (RTI Biologics, Alachua, Florida, USA)
Some biomaterials are cross-linked in order to render the biomaterial less vulnerable to rapid breakdown of collagens and proteins by collagenases and other proteinases. The amount of cross-linking defines the time required for degradation of these biomaterials. For example, 100% cross-linking may render the material impenetrable by host cells and therefore not degradable. The latter goes often hand in hand with encapsulation of the material and isolation from normal tissue turnover. This is not an ideal situation.
Synthetic materials can be classified into absorbable and nonabsorbable, monofilament and multifilament, knitted or woven, microporous and macroporous, and heavyweight and lightweight meshes. Box 27.2 gives some examples of synthetic materials.
Box 27.2
Examples of synthetic meshes
Absorbable: | |
GORE®BIO-A® Tissue reinforcement (polyglycolic acid:trimethylene) | |
carbonate (PGA:TMC) fibers (Gore & Associates, Inc., Newark, Delaware, USA) | |
Nonabsorbable: | |
Gynemesh (polypropylene) (Ethicon, Norderstedt, Hamburg) | |
Smart Mesh (polypropylene, light weight) (Coloplast, Orton, Peterborough, UK) | |
ePTFE (polytetrafluorethylene) (Gore & Associates, Inc., Newark, Delaware, USA) | |
PVDF (polyvinylidene difluoride) (Dahlhausen GmbH & FEG Textiltechnik Aachen, Germany) | |
Parietex Prosup (polyester, large pore, heavyweight)(Tyco Healthcare, Gosport, Hampshire, UK) | |
Mixed, partially absorbable: | |
Vypro® (polypropylene plus polyglactin) (Ethicon, Norderstedt, Hamburg) | |
Ultrapro® (polypropylene plus polyglecaprone-25, large pore, lightweight) (Ethicon, Norderstedt, Hamburg) | |
Mixed, nonabsorbable: | |
Dynamesh IPOM (Polypropylene plus polyvinylidinchloride at abdominal side) | |
(Dahlhausen GmbH & FEG Textiltechnik) | |
Additional surface modifications: | |
Proceed® (Polypropylene plus polydioxanone and cellulose)(Ethicon, Norderstedt, Hamburg) | |
Parietene composite: polypropylene plus collagen/polyethylenglycol/glycerol coating) (Covidien, Mansfield, Massachusetts, USA) |
27.2 Biological Materials
Lyophilized bovine dura mater was used regularly for congenital diaphragmatic hernia repair. The surgical haptic was good, and the material was flexible enough to fit into a dome-shaped form. With the occurrence of BSE (bovine spongiform encephalopathy) and possible transmission of Creutzfeldt-Jakob disease, bovine neuron-related materials were withdrawn from the market after 1992. Despite new avitalization processes, a residual small risk of prion or viral transmission cannot be excluded. Examples of substitutes for neuron-based materials include bovine pericardium [10], porcine pericardium, bowel wall, or fascia; however, the available size of these materials is not always sufficient for repair of large hernias. Large surface area materials, such as dermal matrix or small bowel mucosa, chemically processed and reconstituted, are used for this purpose.
27.2.1 Acellular Biological Materials
Examples of two prototypes of acellular biological materials are discussed: Surgisis® and Permacol®.
Surgisis (Cook Surgical, Bloomington, Indiana, USA) was produced following studies by Hodde et al. [11] describing the development of an extracellular matrix (ECM) made from sterilized porcine small bowel mucosa with its ECM components. Related studies by Cook Surgical postulated complete new tissue regeneration, which was qualitatively similar to the surrounding tissue [12]. As yet, there have been no long-term randomized studies to prove its superior quality. Clinical results comparing Surgisis with other meshes for use in diaphragmatic hernia demonstrated a similarly high recurrence rate of 50% [13]. Our own animal studies demonstrated that 4 months after insertion Surgisis had disintegrated and had been substituted by lower-quality scar tissue, resulting in inferior mechanical quality compared with a polypropylene mesh [14–17]. No improvement of tissue tensile strength was found when comparing animals implanted with Surgisis with control animals without mesh [18]. In a rat model, animals with Surgisis had a much lower tensile strength compared with polypropylene [19].
Surgisis did not show any convincing advantage compared with synthetic materials with respect to seroma formation, adhesions, tensile strength [20], shrinkage [21], and recurrence rates following hernia repair [22].
In summary, the insufficient mechanical strength of Surgisis renders it unsuitable for any application where mechanical support is essential [23], e.g., pelvic prolapse surgery.
Permacol (acellular cross-linked porcine dermal matrix) is another type of biomaterial that is regularly inserted in different hernia locations, including parastomal hernias, and plevic prolapse surgery [24]. Within this group of crosslinked meshes, collagen molecules are covalently bound to each other following chemical processing. This protects the material to a certain degree against degradation by host tissue collagenases. In a study, Permacol showed satisfactory tensile strength over 6 months and better mechanical properties compared with other cross-linked materials [20]. Another study demonstrated a lower hernia recurrence rate with Permacol compared with polytetrafluoroethylene (PTFE) [25]; however, the follow-up time was short and patient numbers were small. In prolapse surgery, clinical short-term results were shown to be promising [26].
However, several recent studies have revealed problems with the use of biological meshes. In a long-term follow-up study using different materials for abdominal incisional hernia repair, Permacol did not show an advantage compared with synthetic meshes [25]. In a clinical study, the recurrence rate of incisional hernia at 18 months using Permacol was 15%, with a complication rate of 35% (e.g., wound infection) [27]. This is no improvement on currently used synthetic meshes. The fistula rate following the use of Permacol intra-abdominally is low, but not unheard of; equally, bowel adhesions with the need for re-intervention have been described [6].
Additionally, Permacol has shown a marked inflammatory response in rats up to 40 days postimplantation, and no ingrowth of skeletal muscle cells [28].
The cross-linked Permacol has demonstrated better mechanical qualities compared with non-cross-linked materials [20], but inferior qualities compared with synthetic meshes. Similar findings were shown in an animal study comparing Pelvicol® with Pelvisoft®, Gynemesh®, and Surgisis. The polypropylene-based Gynemesh showed the highest tensile strength and least stiffness compared with the cross-linked Pelvicol. In addition, Pelvicol showed encapsulation after 3 months insertion time, whereas Gynemesh was found to be incorporated [29]. A clinical study using Surgisis or Pelvicol for sacrocolpopexy at 2 years follow-up found the anatomical recurrence rate to be very high (70%), with a functional recurrence rate of 40% [30].
These results compare very badly with studies on prolapse surgery using synthetic meshes. On the other hand, long-term clinical follow-up results on the use of synthetic mesh for laparoscopic anterior rectopexy for rectal prolapse describe very low recurrence rates of no more than 5% [31, 32].
Additionally, the hope for superior behavior of biological materials within a contaminated area is contradicted by multiple studies. Following inoculation of meshes with bacteria, findings have indicated a possible higher tendency of infection when using biological materials [33]. Additionally, inoculation seems to weaken the tensile strength of the biological mesh [34].
In summary, biological materials have not shown convincing superiority compared with synthetic nonabsorbable meshes in hernia repair [35, 36], or in prolapse/pelvic floor surgery [37–39]. Another disadvantage with biomaterials is their inconsistent ECM composition (e.g., type and amount of collagen). Therefore, their functional outcome is not predictable, plus, this inconsistency renders them unsuitable for surface modulation.
27.3 Synthetic Meshes
Synthetic meshes are frequently used in hernia surgery, in the knowledge that they produce good long-term results. Typical qualities include good biocompatibility, reproducibility, and consistency. The concept of lightweight and heavyweight meshes is well established [40]. The basis for this concept was the finding that the host inflammatory response depends on the material density and pore size of the mesh construct. A lightweight and large-pore-size mesh is more suitable than a heavyweight and small-pore-size mesh.
However, a limited inflammatory reaction is tolerated and is required for tissue remodeling and adequate scar formation [41]. Therefore the density of the material was reduced and the pore size was enlarged, which led to optimal tissue incorporation of the mesh and avoidance of biofilm production [42–45], with no impact on the good mechanical qualities of the material [46–49].
Mesh shrinkage of synthetic meshes is in the range of 3–30%, depending on their location, textile structure, and weight [50]. In hernia surgery, this is compensated by mesh overlap.
For the intra-abdominal application of meshes, anti-adhesive materials are added to the mesh. For example, Proceed® has a cellulose cover on its abdominal side [51, 52]. Studies comparing polypropylene meshes with different covers suggest that there is room for further improvement [52, 53].
Ultrapro® (polypropylene plus polyglecaprone-25) has demonstrated superior biocompatibility in animal studies in comparison with other synthetic materials [54], and is one of the most frequently used materials in hernia surgery [55].
PTFE was known for its lack of elasticity and tendency toward encapsulation and failed tissue integration [21]. It was hoped that there would be an improvement in these properties in the expanded version of PTFE: ePTFE. In one study, it showed fewer adhesions compared with polypropylene in the intra-abdominal position, but it also showed a shrinkage rate of 30% [51].
27.3.1 Surface-modulated Meshes
The search for the ideal mesh is ongoing. The advantage of synthetic meshes is the possibility of modifying the surface of currently available commercial meshes (Fig. 27.1) [56–59]. It is possible to add active agents such as antibiotics, protein- repellent substances, and inflammatory response proteins. Also, one can configure a three-dimensional scaffold that allows cell inoculation according to the required needs. Ongoing in vitro research is attempting to establish a system in order to test cell interaction with different biomaterials [60–64]. Controlled release of active substances (e.g., antibiotics, cytokines, growth hormones) bound to the mesh is also easily achieved [65].
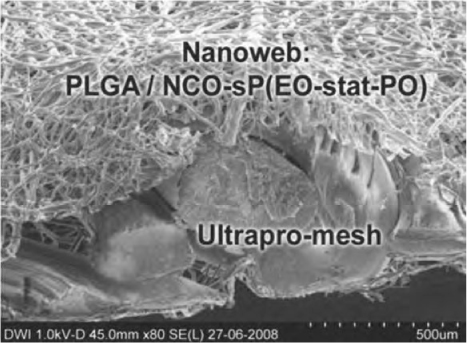

Fig. 27.1
Electronmicroscopy of a surface-modified mesh: electrospun absorbable nanoweb on Ultrapro® mesh. PLGA (polylactide glycolic acid) with NCO-sP(EO-stat-PO) as a possible carrier for active substances
27.4 Summary
In summary, the individual needs of the patient, the target location and its required function, and the insertion route [66] plus its surrounding communicating tissue [67] will decide whether a biological or synthetic, absorbable or nonabsorbable material, is most suitable.
The main advantage of synthetic material is its precise reproducibility, absent infectious risk, and possibility for surface modification, and addition of active agents such as inflammatory modulators or stem cell inoculation. Mechanical qualities of different synthetic meshes have been studied and the surgeon has to decide whether a stiffer or more elastic material is needed. For prolapse surgery, a more elastic mesh such as Ultrapro or smart mesh seems advisable, considering the proximity to the rather vulnerable vaginal tissue [67]. In order to avoid mesh erosion into or constriction of viscous organs (rare, but devastating for the patient when it happens) we would recommend use of as little implant material as possible, never surrounding an organ completely, and covering the material with a good amount of the patients’ own tissue as a barrier. These recommendations are fulfilled, for example, in the anterior rectocolposuspension technique described by D’Hoore and Penninckx [68], using a small strip of mesh and covering it completely with peritoneum and fat.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






