Fig. 12.1
Anatomy of anal sphincters and puborectalis muscle. Red puborectalis muscle, yellow external sphincter deep portion, pale yellow external sphincter superficial portion, orange internal sphincter
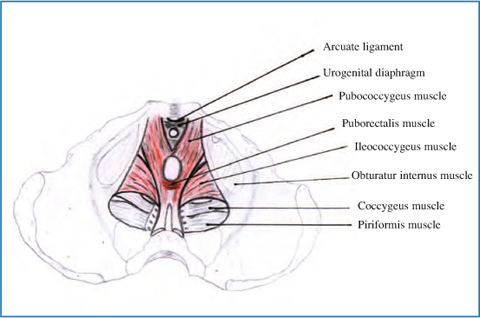
Fig. 12.2
Anatomy of levator ani complex
Concerning the diagnosis, careful perineal and digital rectal examination, colonic transit time study, anorectal manometry, anal electromyography (EMG) defecography, or dynamic magnetic resonance imaging (MRI) of the pelvic floor usually help to assess a correct diagnosis.
Anorectal manometry provides a comprehensive assessment of anal pressures, rectoanal reflexes, rectal pressures, sensation, and compliance. Several types of recording devices are available, but perfused catheters and balloon probes are among the most commonly used. A paradoxical increment in anal pressure on straining efforts is a distinctive feature of dyssynergic defecation [4] (Figs. 12.3 and 12.4). An increment in muscle motor activity on straining may be demonstrated by means of EMG either by intra-anal electrodes or by electrodes taped to the perianal skin.
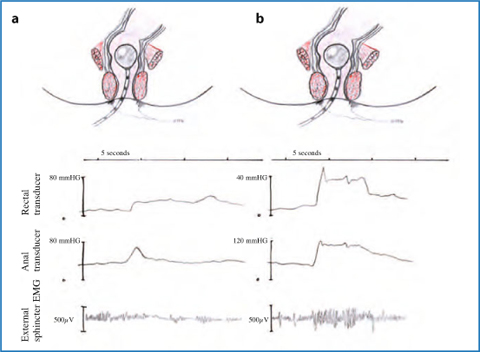
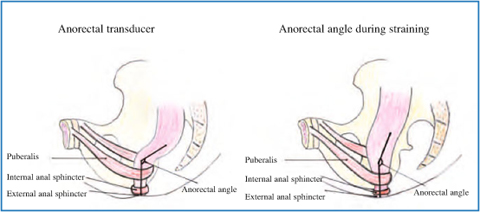

Fig. 12.3
Anal manometry and electromyography during defecation in a normal person (a) and in presence of pelvic floor dyssynergia (b)

Fig. 12.4
Anorectal angle at rest and during straining in absence of pelvic floor dyssynergia
Defecography is a radiographic test providing morphological and functional information on the anorectum. Several parameters may be assessed, such as pelvic floor descent, anorectal angle, rectocele, and rectal prolapse. Failure of the anorectal angle to become more oblique on straining provides indirect evidence of defective pelvic floor relaxation, and impaired evacuation of contrast material is also suggestive of dyssynergia [4].
Anal EMG may be recorded either by intra-anal probes or by perianal EMG electrodes attached to the skin [6, 7]. The EMG activity used in biofeedback training is the averaged activity of large numbers of muscle cells rather than the activity of small groups of muscle cells innervated by a single axon. This averaged EMG activity is recorded with large electrodes on the skin or the mucosa of the anal canal rather than with needle electrodes. Averaged EMG recorded in this way is proportional to the strength of contraction of the underlying muscles (Fig. 12.4).
Defective expulsion is commonly investigated by asking the patient to defecate a 50 ml water-filled rectal balloon; patients with functional defecation disorders usually fail this test [7]. Some patients also have a higher threshold for perceiving the urge to defecate [6], but the clinical significance of this sensory dysfunction is ill-defined, in contrast to the relevance of rectal sensory impairment in fecal incontinence [8].
Patients with functional defecation disorders are often unresponsive to conservative medical management, and the surgical division of the puborectalis muscle (which has been proposed for the treatment of dyssynergic defecation) has resulted in poor benefit and an unacceptable risk of anal incontinence [1, 3]. Treatment with botulinum toxin injection may provide temporary improvement, but it remains an investigational treatment. Therefore, the gold standard of therapy of pelvic floor dyssynergia is conservative and is based on a high-fiber diet, physical activity, and biofeedback training [2].
12.1.2 Biofeedback Techniques for Pelvic Floor Dyssynergia
Biofeedback is a conditioning treatment where information about a physiologic process (contraction and relaxation of a muscle) is converted to a simple visual or auditory signal to enable the patient to learn to control the disordered function. Biofeedback is considered appropriate when specific pathophysiological mechanisms are known and the voluntary control of responses can be learned with the aid of systematic information about functions not usually monitored at a conscious level [6]. Biofeedback involves the use of pressure measurements (manometry) or averaged EMG activity within the anal canal to teach patients how to relax pelvic floor muscles when straining to defecate.
Various biofeedback techniques (intra-anal and perianal EMG monitoring, manometric anal probe biofeedback, intra-rectal balloon expulsion biofeedback, ultrasound biofeedback) have been investigated, but none has a superior success rate, which ranges from 30% to over 90% [9–11].
Despite controversy about the method of biofeedback and the number of sessions needed, it seems reasonable to use this generally safe technique as the initial treatment for pelvic floor dyssynergia.
Biofeedback training protocols vary among different centers [6, 7]. A mainstay of behavior therapy is to first explain the anorectal dysfunction and discuss its relevance with the patient before approaching the treatment [3, 7]. Biofeedback interventions for pelvic floor disorders are directed at teaching patients to relax their pelvic floor muscles while simultaneously applying a downward intra-abdominal pressure to generate propulsive force (Valsalva maneuver). Patients are shown anal manometry or EMG recordings displaying their anal function and are taught through trial and error to relax the pelvic floor and anal muscles during straining [6–8]. This objective is first pursued with the help of visual feedback on pelvic floor muscle contraction, accompanied by continuous encouragement from the therapist. When the patient has learned to relax the pelvic floor muscles during straining, the visual and auditory help are gradually withdrawn [6]. Another retraining option is to simulate defecation by means of an air-filled balloon attached to a catheter, which is slowly withdrawn from the rectum while the patient concentrates on the evoked sensation and tries to facilitate its passage [3, 7]. In the next phase of training, the patient is taught to defecate the balloon by bearing down, without the assistance of the therapist. Some centers also add a balloon sensory retraining to lower the urge perception threshold [12]. The number of training sessions is not standardized, but 4–6 sessions are frequently provided. Individual training sessions last 30–60 min.
Therapeutic sessions are professionally demanding, and a highly trained and motivated therapist is essential. No study has addressed the necessary training required for an individual to administer biofeedback therapy. In particular, it is unclear whether the adequate provider should be a physician, psychologist, or nurse. Experience varies among centers, but the low cost reimbursement provided for behavior therapy is likely to influence future choices.
12.1.3 Biofeedback for Pelvic Floor Dyssynergia: Literature Results
Controlled studies systematically comparing different biofeedback protocols with each other are lacking. In the literature, the symptomatic improvement rate has been shown to vary widely between 44% and 100% in several uncontrolled clinical trials. Subsequent randomized trials have confirmed the higher efficacy of biofeedback compared with standard therapy and laxatives.
Chiarioni et al. [13] randomized 109 dyssynergic patients to EMG biofeedback training or to polyethylene glycol and assessed outcomes at 6 and 12 months. Biofeedback patients were more satisfied than the control group (80% vs. 22%, p < 0.001) and reported greater reductions in blocked or incomplete bowel movements, straining, abdominal pain, and use of enemas and suppositories. Stool frequency increased in both groups.
Rao et al. [14] compared anal pressure biofeedback to two control conditions – sham biofeedback and standard care – in 77 patients. Standard care subjects received diet and life style advice, laxatives, and scheduled evacuations. At 3 months follow-up, the biofeedback reported significantly more complete spontaneous bowel movements, defecation improvement, and higher satisfaction than the sham-treated group.
Heymen et al. [15] compared EMG biofeedback to two control conditions: diazepam (5 mg) (a skeletal muscle relaxant) or placebo 1 h before attempted defecation. Prior to randomization, all 117 patients were provided with enhanced standard care that included diet, lifestyle measures, stool softeners, and scheduled evacuations during a 4-week run-in and those who reported adequate relief at the end of run-in (n = 18, 15%) were excluded. Eighty-four patients were randomized. At 3 months follow-up, biofeedback-treated patients reported significantly more unassisted bowel movements than placebo-treated patients (p = 0.005) In the intent-to-treat analysis, 70% of the biofeedback group reported adequate relief compared with 23% of diazepam-treated patients (p < 0.001) and 38% of placebo-treated patients (p = 0.017).
Although it is encouraging that more controlled studies have been carried out in recent years, these trials were heterogeneous with regard to inclusion criteria, treatment protocols, and end points.
The mechanism of action of biofeedback therapy is also not known. Although various parameters of colonic and anorectal function show improvement, and one study showed improvement in distal colonic blood flow [16], the precise alterations are unclear. Recent studies using bidirectional cortical-evoked potentials and transcranial magnetic stimulations suggest significant bidirectional brain-gut dysfunction in patients with dyssynergic defecation [16]. Whether an improvement in bowel function correlates with an improvement in brain-gut dysfunction remains to be explored.
Finally, concerning the technique of choice, a recent meta-analysis showed that in open-label studies, the mean success rate with pressure biofeedback was slightly greater than with EMG biofeedback (78% vs. 70%) [17]. No differences were found between anal versus perianal EMG recording. In addition, adding balloon feedback did not seem to influence the therapeutic outcome [17]. However, the majority of studies in the last 10 years have utilized EMG biofeedback rather than pressure feedback, even in the absence of scientific evidence [17]. There are no standardized protocols, and centers use different combinations of laboratory EMG training, home EMG training, and balloon feedback, depending on the researchers’ experience.
12.2 Biofeedback Therapy for Fecal Incontinence
12.2.1 Fecal Incontinence
Fecal incontinence (FI) is a debilitating and embarrassing problem facing approximately 2.2% of the US general population over 65 years old. The etiology of FI is multifactorial and can be due to several factors including neuropathic, traumatic, congenital, and obstetric trauma, as well as iatrogenic injuries caused by injudicious fistula surgery, hemorrhoidectomy, and lateral internal sphincterotomy, among others [18]. FI symptoms can range from mild to severe and the work-up and treatments of this disorder are just as varied. Patients may complain of incontinence to flatus, or liquid or solid stools. In some patients, just the concern that an accident may happen adversely affects their daily quality of life and limits their ability to interact socially due to fear and embarrassment. Several scoring systems have been created and validated to help patients and their medical practitioners quantify the severity of symptoms and the effects of FI on their daily life. These scores are used by physicians to plan treatment strategies and by researchers to study the outcomes of FI treatments [18].
The mechanism of fecal continence is extremely complex despite the simplicity that physicians often ascribe to it. The sphincter mechanism requires the ability to discriminate between solid, liquid, and gas; voluntarily allowing for the passage of one while holding the other components [19]. Treating FI requires an understanding of this complex pelvic floor musculature, innervation, and function, as well as the mechanisms that must be present to ensure continence. The internal and external sphincters and the puborectalis muscle comprise the sphincter mechanism. The internal anal sphincter (IAS) is a continuation of the circular, smooth, involuntary muscle of the rectum that accounts for the resting tone of the anus. The rectoanal inhibitory reflex allows the internal sphincter to relax in response to rectal distension, preparing the anal canal for defecation [18, 19]. The external anal sphincter (EAS) provides voluntary control over defecation and provides the squeezing pressure measured by anal manometry. The puborectalis is a U-shaped muscle that controls the rectoanal angle that increases during defecation. Both parasympathetic and sympathetic nerves provide the innervation of this sphincter complex. The pudendal nerve innervates both the puborectalis and EAS and when neurogenic incontinence is present, latency of this nerve can be detected [19]. Because of the embarrassing nature of FI, symptoms are often hidden by patients and thus are underreported and undertreated. Once these symptoms are voiced, it is important to obtain a detailed account of the incontinence. Descriptions of partial incontinence to only gas or liquid stools, and occasional or complete involuntary passage of solid stools should be provided to assess severity. Episodes of soiling or leakage and use of protective pads for undergarments are important, as well as the thorough assessment of general bowel habits. A careful history of anorectal surgery, colorectal disease, anal intercourse, obstetric trauma, rectal prolapse, and neurologic disorders should be taken.
Details of the patient’s stool frequency, consistency, or frequency of incontinent episodes should be obtained to assess the severity FI symptoms. There have been several score indices created to quantify symptoms: the Fecal Incontinence Severity Index (FISI), or the Cleveland Clinic Incontinence Score that combines the loss of flatus, liquid, and solid stools as well as impact of quality of life to assess the severity of FI. Other scoring systems specifically address the effects of FI on quality of life, as in Fecal Incontinence Quality of Life Questionnaire (FIQL) published by the American Society of Colon and Rectal Surgeons. The clinician can use these tools to assess the severity of symptoms and thus recommend a strategy for evaluation and treatment [19].
Regarding the diagnostic tests [20], the first step is to identify whether the incontinence is secondary to diarrhea. If so, endoscopic mucosal evaluation, stool tests, and breath tests may be useful. Anorectal manometry quantifies IAS and EAS function, rectal sensation, rectoanal reflexes, and rectal compliance. Anal endosonography provides an assessment of the thickness and structural integrity of the EAS and IAS and can detect scarring, loss of muscle tissue, and other local pathology. It is performed by using a 7–12 mHz rotating transducer with a focal length of 1–4 cm. More recently, three-dimensional ultrasound imaging has become available, which provides better delineation of anal sphincters and puborectalis and surrounding structures Rectal balloon distention with incremental volumes of air can be used for the assessment of both sensory responses and compliance. Incontinent patients may exhibit rectal hyposensitivity or hypersensitivity. MRI provides superior imaging with better spatial resolution of the EAS. A major contribution of anal MRI has been the recognition of external sphincter atrophy, and sometimes without pudendal neuropathy. The addition of dynamic pelvic MRI by using fast imaging sequences or MRI colpocystography, which involves rectal filling with ultrasound gel and having the patient evacuate while lying inside the magnet, may each provide better delineation of pelvic anatomy. The use of an endoanal coil significantly enhances the resolution and allows more precise definition of sphincter muscles. However, comparative studies with other technology, and costs and clinical utility have not been assessed. EMG of the anal sphincter identifies sphincter injury as well as denervation-reinnervation potentials that indicate neuropathy. Finally, the integrity of the entire spino-anorectal pathways that control anorectal function can be assessed by magnetic stimulation and recording of motor-evoked potentials [19, 20].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






