Fig. 1
Schematic of Paris classification of superficial neoplasia and corresponding endoscopic images examples of common AMN morphologies encountered. Lesions are broadly divided into polypoid (0-I) and flat (0-II) morphologies on the basis of protrusion > 2.5 mm above the mucosal surface in 0-I lesions in contrast to the lower vertical height of 0-II lesions. The margin of 0-IIb lesions or the depressed area within nongranular flat lesions may be indistinct and is best appreciated in these cases under narrow-band imaging (NBI). Mixed morphologies may exist with an elevated risk of SMI associated with depressed areas (0-IIc) or flat lesions with large nodule (0-IIa + 0-Is)
An additional descriptor for lesions larger than 10 mm in diameter demonstrating a predominately horizontal pattern of growth with a low vertical trajectory is laterally spreading tumor (LST). LSTs are classified as 0-IIa or 0-IIb by elevation criteria under the Paris classification [22]. Adenomatous (either conventional or traditional serrated adenomas) may be further categorised by their surface granularity as granular (G), non-granular (NG) (Fig. 2) [32]. Although hyperplastic and sessile serrated adenomas/polyps may often exhibit the morphology of LSTs, they are not described within this system [32] and the approach to these lesions is described later.
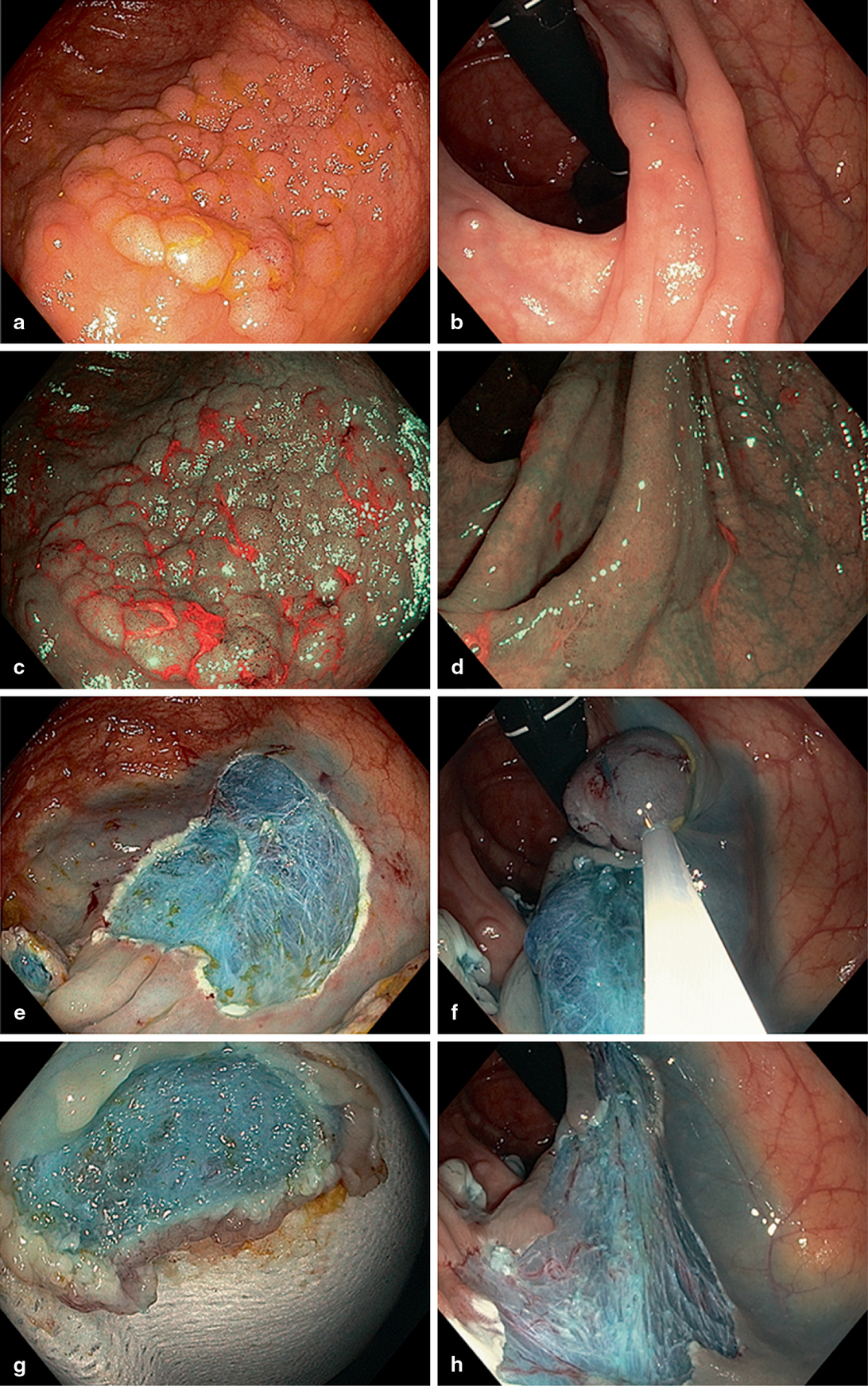

Fig. 2
Series of comparative images of granular and non-granular homogenous LSTs in the proximal colon. Thirty millimetres caecal granular LST (Paris 0-IIa) WL (a) and narrow-band imaging (NBI) (c). Piecemeal resection defect (e) and underside specimen (g) both demonstrating homogenous blue submucosal (SM) staining. Thirty-five millimetres ascending colon nongranular LST (Paris 0-IIb) with subtle appearance under WL (b) and enhanced margin visualisation under NBI (d). Sequential resection (f) and resultant defect (h)
Association Between Morphology and Risk of SMI
LST-G (0-IIa and 0-IIa + Is) are prevalent in cohorts of colonic polyps referred for resection and overall represent a low rate (3.2 %) of SMI in this setting [15]. A homogeneous 0-IIa LST-G has a risk of SMI of approximately 1 % [15, 33, 34]. They are ideal for endoscopic therapy even when very large. A sessile component in a flat elevated lesion (0-IIa + 0-Is) has a higher risk of SMI at 6–10 % [33]. LST-NG and LST-G are biologically distinct with different rates of oncogene expression [33, 35]. The NG pattern represents a higher risk of SMI which ranges from 5 to 15 % in a homogeneous lesion up to 70 % in those with a depressed area [15, 32, 33, 36]. In the majority of cases, the area of SMI lies under the Is nodule or depressed area which allows targeting of this area for initial resection [33].
Surface Pattern
The surface of a lesion may be assessed either in terms of pit (Kudo pit pattern) [37] or vascular pattern (multiple classifications) [38]. Kudo’s anatomic pit pattern was described with magnifying colonscopes and chromoendoscopy [37] and has been utilised for prediction of depth of invasion [39, 40]. In summary type I and II are typical of normal mucosa or hyperplastic lesions, type IIIL and IV of adenoma and IIIs and Vi with high-grade changes or SMI < 1000 μm. Vi pattern within a demarcated area or Vn pattern is associated with deeply invasive adenocarcinoma [41–43].
Surface microvascular classification is based on the observation that tumourogenesis involves angiogenesis with altered microvessels [44, 45], which may be better appreciated with push-button modalities such as narrow-band imaging (NBI). The central concept is that with advancing histological grade vessels become thicker and more tortuous before then becoming irregular and obliterated. In the strictest sense, NBI does not allow assessment of the Kudo pit pattern [38], but rather demonstrates a pit-like pattern. Given the diversity of NBI classifications (Sano, Hiroshima, Showa, Jikei and additional Japanese University classifications) [38] a unified classification which does not require magnifying colonoscopes has been proposed (NBI International Colorectal Endoscopic (NICE) classification) [38, 46, 47]. Classifications have significant predictive capability; however, in the absence of magnifying colonoscopy evidence is predominately limited to discrimination between adenomatous and serrated polyps [48–51] rather than identifying SMI [38, 42, 52, 53]. Further studies validating both pit and vascular classification in predicting SMI are required. Surface pattern assessment is more accurate in flat and sessile lesions rather than pedunculated lesions [54] and is additive when combined with morphology, for example, NG-LSTs with depressed (0-IIc) area and type V Kudo pit pattern has predictive power of 50 % for invasive disease (Fig. 3), in the hands of Western endoscopists without magnification [15].
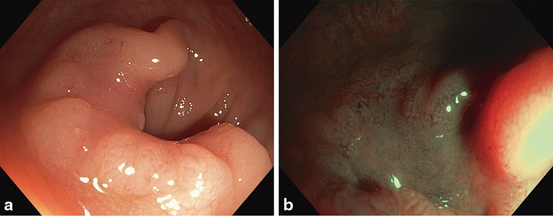

Fig. 3
a 30 mm nongranular LST with depression (0-IIa + 0-II c). b Loss of regular dark vascular pattern in the depressed area in non-magnified NBI view highlighting irregular to absent vascular pattern and Vi-n pit-like pattern. Biopsies taken after non-lifting demonstrated high-grade dysplasia; histopathology of the surgical specimen confirmed adenocarcinoma with deep submucosal invasion (SMI)
If deeply invasive disease is predicted endoscopically, our practice is to perform targeted biopsies, place a localising tattoo if required and refer the patient for staging imaging and surgery. Multiple tunnelling biopsies should not be taken when a large flat lesion is first encountered if it is potentially considered suitable for endoscopic resection basedon endoscopic features due to the risk of SM fibrosis and subsequent non-lifting.
The WF-EMR Procedure
Pre-Procedural Preparation
Although no guidelines exist on the consent for WF-EMR, risks, alternative therapies and consequences of no action should be conveyed [55]. Specific consent reflecting the increased risks of WF-EMR of AMN over those of routine colonoscopy and polypectomy should be obtained. All patients including those with distal sigmoid or rectal lesions should receive full bowel preparation to facilitate optimal views and mitigate against the risk of contamination in the event of perforation. Carbon dioxide (CO2) insufflation reduces colonoscopy procedural and post-procedural discomfort [56, 57] and post-procedural admission after WF-EMR [58] in a large prospective cohort study.
Guidelines for management of anti-platelet therapy prior to WF-EMR do not exist. General guidelines for endoscopic procedures are a useful template [59, 60]. However an individualised approach to peri-procedural anti-platelet and anti-coagulation management should be employed and the use of these agents must always be factored into the therapeutic approach. Cardiologist consultation should be considered before anti-platelet or anti-coagulation discontinuation in patients with coronary stents or metal prosthetic valves. If a compelling indication for ongoing anti-platelet therapy exists, we continue with low-dose aspirin in preference to alternative anti-platelet agents. Enoxaparin window technique may be used for prosthetic valves with the drug omitted on the day of the procedure only. The post-procedural bleeding risk is significant but generally amenable to therapy, and must be balanced against the more serious risk of cardiac thromboembolism which is frequently catastrophic.
EMR Procedure
The colonoscope position for resection should be optimised prior to commencement. Reduce loops to the shortest most stable scope position. Rotate the colonoscope so that the lesion lies at the 5–6 o’clock position; however, this should not be considered mandatory, if an ideal resection aspect exists at an alternative position. Wash, inspect and photo-document whilst assessing morphology and interrogating surface pattern. Complete assessment or optimal resection position may require retroflexion, particularly in the distal rectum or ascending colon/hepatic flexure. Routine retroflexion with an adult scope is achievable and safe in the majority of patients in these locations [61]. If colonic spasm is problematic intravenous hyoscine bromide 10–20 mg or glucagon 0.5–1 IU may be helpful.
Injection
SM injection is a critical component of WF-EMR. The ideal injectate should be safe, affordable and provide a sustained and localised elevation when injected into the SM space [62, 63]. Normal saline is most commonly used; however, it may disperse along the SM space limiting the magnitude and duration of elevation. Alternatives include: glycerol, hyaluronic acid and succinylated gelatin. Fluid selection is influence by local availability and cost [63, 64]. In randomised trials, succinylated gelatin increases ER en bloc resection size in a porcine model [65] and reduces procedure duration and number of resection pieces in humans [62]. Dilute indigocarmine is added to the injectate. This dye is avid for the loose areolar tissue of the SM. The benefits are threefold.
1.
Delineate the area of successful SM injection where resection may be safely performed.
2.
Highlight the margin of the lesion especially of indistinct and flat neoplasms, for example, 0-IIb NG lesions.
3.
Stain the mucosal defect creating a homogenous blue mat expanse of obliquely orientated SM fibres. This facilitates the evaluation for deep injury.
Methylene blue is an alternative dye; however, it does not perform as well. Epinephrine (adrenaline) is added with the aims of reducing minor intra-procedural bleeding (IPB) to facilitate a clear endoscopic view and to reduce dispersion of the SM injectate through vasoconstriction of draining vessels [14]. Its effect upon post-polypectomy bleeding is unclear [66–68]. Practically, we add 80 mg of indigocarmine to a 500 ml bag of succinylated gelatin (Gelofusine ® B. Braun Australia), 9 ml of this solution is drawn into a 10 ml syringe with 1 ml of 1:10,000 epinephrines (adrenaline) in normal saline.
Injection Technique
Approach the mucosa at an angle of approximately 30 %. A tangential approach reduces the risk of trans-mural injection. Use the ‘touch and inject’ technique, where the injecting catheter with the needle ‘out’ is advanced until it touches the mucosa. The assistant then begins injection whilst simultaneously the endoscopist advances the catheter rapidly with a short ‘stab’. This method rapidly finds the SM plane. Immediate elevation should occur. If elevation is not seen deep injection has likely occurred. Unless excessive this is inconsequential, and the needle should be slowly withdrawn till satisfactory mucosal elevation occurs. Whilst elevation of the entire lesion is essential for en bloc resection ; for WF-EMR, this may hamper piecemeal resection due to an excessive tension within the fluid cushion limiting tissue capture within the snare. It may also crowd the lumen restricting access to the whole lesion. Tissue elevation tends to be transient so repeat injection is necessary in any case. Whilst injection occurs, the catheter and scope may be gently moved as one to ‘guide’ the formation of the tissue elevation in the desired direction [14, 69, 70]. Translesional injection should be avoided, if SMI is suspected.
Electrosurgical Device
In contrast to pedunculated lesions, where a low-power coagulation current is used to coapt feeding vessels, for ER of flat or sessile lesions more sophisticated electrosurgical outputs are required. Microprocessor (e.g., ERBE VIO 300, Tubingen, Germany, Olympus ESG-100, Tokyo, Japan) controlled alternating cut and coagulation cycles with automatic adjustment of power output in relation to tissue impedance changes during polypectomy are preferable [14]. This approach is based on sound electrophysiological principles, but is unproven in clinical trials.
Snare Selection
A range of snares is required. We utilise a 20 mm spiral snare for most WF-EMR cases. The serrated wire aids tissue capture, crucial to the inclusion of a margin of normal mucosa in the resection. A small thin-wire snare should be available for resection of residual disease within and at the margin of the defect. It is also useful for areas with fibrosis, poor lifting or difficult access. Multiple snare types with varied shape and size on opening and different wire characteristics are available and may be utilised in specific circumstances largely guided by proceduralist preference.
Resection
En bloc resection is ideal; however, it is limited by lesion size and location with current ER techniques. During piecemeal ER each resection has the potential to create complications and leave disease; hence, the aim should be safe resection in the fewest number of pieces possible (an oligo-piecemeal resection). Start resection in the area most likely to contain SMI if present, e.g., the Is component of a IIa + Is lesion, a IIc component of an LST-NG lesion or demarcated area with advanced pit or surface pattern (Fig. 4). For homogeneous lesions commence at the area anticipated most challenging to resect. After injection the lesion;
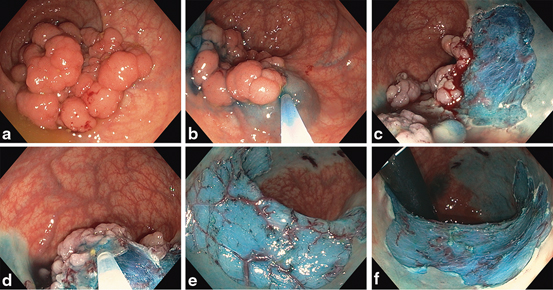

Fig. 4
a 50 mm hemi-circumferential rectal granular LST (0-IIa + 0-Is). Submucosal (SM) injection b then resection c of distal margin. d Injection of central portion. Hemi-circumferential mucosal defect with prominent non-bleeding vessels on forward e and retroflexion f views.
Work sequentially from the point of first entry into the SM plane. Align the snare with the edge of the advancing mucosal defect and continue this technique as the lesion is removed. This reduces the risk of tissue islands (within the defect), which are difficult to subsequently remove.
With the snare open over the lesion push down very firmly with the up-down control onto the SM cushion whilst aspirating gas to; reduce colonic wall tension, decrease the mucosal footprint of the lesion, and maximise tissue capture.
Perform a staged snare closure, advancing the catheter to maintain the snare base at the lesion edge, whilst monitoring the lesion for ‘buckling’ or loss of the margin, which requires snare repositioning.
Close the snare very tightly to exclude muscularis propria (MP) from the captured tissue. If using a spiral (serrated) snare, it is not possible to transect ensnared tissue of more than 10 mm diameter without the use of diathermy.
Tent the captured tissue away from the wall of the colon. If concern for MP entrapment exists at this point, slightly open the snare so the MP can ‘drop out’. This technique can also be used, if an adjacent fold has been inadvertently entrapped.
The proceduralist should take the snare for the final transection phase. The sensory feedback is invaluable to inform on the safety and efficacy of the excision. Safe tissue capture is confirmed by three manoeuvres:
Assess the mobility of the ensnared tissue by moving the snare catheter quickly back and forth; it should move freely relative to the underlying colonic wall.
The snare should close fully with minimal ‘puckering’ of the surrounding tissue. If concerned once again the MP release manoeuvre can be repeated as before. The snare is, thus, partially opened and tented into the lumen to release the deeper tissue before repeat closure.
Transection should be fast; the snare is kept tightly closed whilst the foot pedal is depressed. With a microprocessor-controlled generator, using alternating cycles of high-frequency short-pulse cutting on a background of coagulation current, generally between one and three pulses, but occasionally up to 5–6, to transect the tissue. A longer transection phase raises concerns for either the MP entrapment or deeper neoplastic invasion [71]. These features are less reliable in the presence of SM fibrosis or with the scope in retroflexion.
The defect is washed and examined after each resection for evidence of deep injury or residual tissue. NBI and topical SM chromoendoscopy (TSC) [72] are used to evaluate potential areas of residual adenoma, MP injury or non-staining.
Residual tissue may remain, particularly at resection margins. We attempt complete snare excision of all endoscopically visualised polyp with a margin of normal tissue wherever possible. Minute areas can be difficult to ensnare even with smaller thin wired snares and tissue ablation may be required. The use of argon plasma coagulation (APC) has been studied in two settings. Firstly, when applied to a defect which appears to have had all tissue resected has been shown to reduce residual/recurrent tissue at surveillance in a small cohort of lesions > 15 mm [73]. The second setting is use of APC to endoscopically visible disease not amenable to snare resection , where if no therapy is initiated persistent disease is apparent in 100 % of patients at surveillance [74]. When APC is applied to endoscopically visible residual tissue, the described recurrence rates are disappointingly high: 14 [75], 39.5 [15], 46.5 [73], 47.5 % [76] and 50 % [74] indicating it has limited efficacy in this setting. The optimal treatment modality for this type of residual disease is not known.
Risk factors for primary resection failure on multivariate analysis are as follows: previous attempt at resection, ileocaecal valve (ICV) involvement and difficult position, as defined by the proceduralist [15].
Specimen Retrieval and Assessment
When several resection fragments exist a net is used. For very large lesions, the scope may need to be withdrawn and reinserted. The underside of the retrieved fragments is assessed quickly ex vivo, preferably with the colonoscope, for evidence of a target sign. Large pieces are pinned flat prior to submission for histopathologic examination. En face ex-vivo interrogation with the enhanced imaging functions of the colonoscope confirms the primary endoscopic assessment. These specimens are submitted separately. As the en face view maybe superior to the in vivo endoscopic image, this type of controlled analysis is a convenient means of improving the endoscopists imaging skills. We undertake extensive photo-documentation with subsequent histological correlation to improve our imaging skills and diagnostic accuracy. Smaller fragments collected from the suction channel via a trap or gauze are also submitted.
Lesion Localisation
The site of the lesion should be readily localised so surveillance colonoscopy or if necessary, laparoscopic surgery, can be performed. For rectal lesions, record the distance with a straight scope, from the distal margin of the lesion to the anal verge. Lesions in the caecum or very proximal ascending colon may be described in relation to the ICV referenced at the 9 o’clock position on the medial wall. For other regions of the colon, tattoo placement should be considered. Sterile carbon suspension is not biologically inert and can be associated with complications [77, 64]. Fibrosis may occur when tattoo tracks to the site of a lesion or resection site, resulting in challenging resection [64]. Unintended transmural injection has been associated with phlegmon formation and peritoneal staining making localisation at surgery difficult. Guidelines on colonic tattoo have been proposed [64]; tattoo with sterile carbon should be placed 3 cm distal to the lesion/defect with saline pre-injection technique. The injectate used for the preceding ER may also suffice. An injection technique identical to that described above for lesion elevation is employed. When elevation occurs the saline/injectate syringe is exchanged for the tattoo syringe and 3 ml of tattoo solution injected. A switch back to the saline syringe and injection of a further 2 ml will clear the injection catheter of the remaining tattoo and allow rapid progression to pre-injection at the second tattoo site. A minimum of two tattoos with the second on the contralateral wall is recommended for surgical localisation.
Limited Elevation with SM Injection
A failure to achieve elevation during SM injection, despite the needle situated within the correct anatomic layer, is known as the non-lifting sign. An analogous sign may be demonstrated, where a stream of injectate exits the area for elevation at the same speed as it is injected, the ‘jet sign’ [14] (Fig. 5). These signs indicate that the SM space has been obliterated usually by fibrosis or uncommonly by direct carcinomatous involvement. In 2013, the application of correct enhanced imaging strategies should avoid the clinical scenario where a deeply invasive disease is attempted to be elevated. Fibrosis may be induced by mechanical or thermal injury from previous resection attempts, reaction to tattoo [64] placed for localisation or reaction to SM tumour invasion. The accuracy of the lifting sign in discriminating between lesions with or without SMI is often misconceived. Several studies [78–80] have evaluated the lifting sign in colonic lesions; overall adenomatous lesions and adenocarcinoma with SMI limited to ≤ 1000 μm (i.e., SM1) generally lift. Deep invasion beyond 2000 μm is generally not associated with lifting; however, lesions with intermediate depth of SMI (1000–2000 μm), which is beyond the current criteria for ER, may lift [81]. Thus, a lesion with concerning morphology and or surface pattern may lift for resection yet ultimately return histology with SMI depth requiring surgical management (Fig. 6). Conversely, if a lesion fails to lift in the absence of prior significant biopsy or resection attempts, deep invasion is possible particularly, where Is morphology is present as this may conceal cancer deep within the lesion. Where there is a history of resection attempts or multiple biopsies in a flat lesion and this histology does not indicate SMI, attempts at elevation and resection of a poorly lifting lesion can be considered and is discussed below.
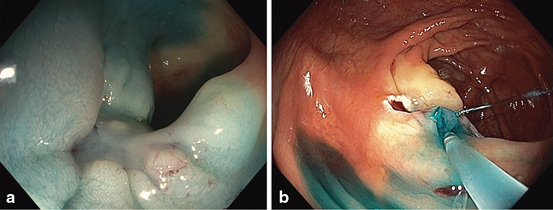
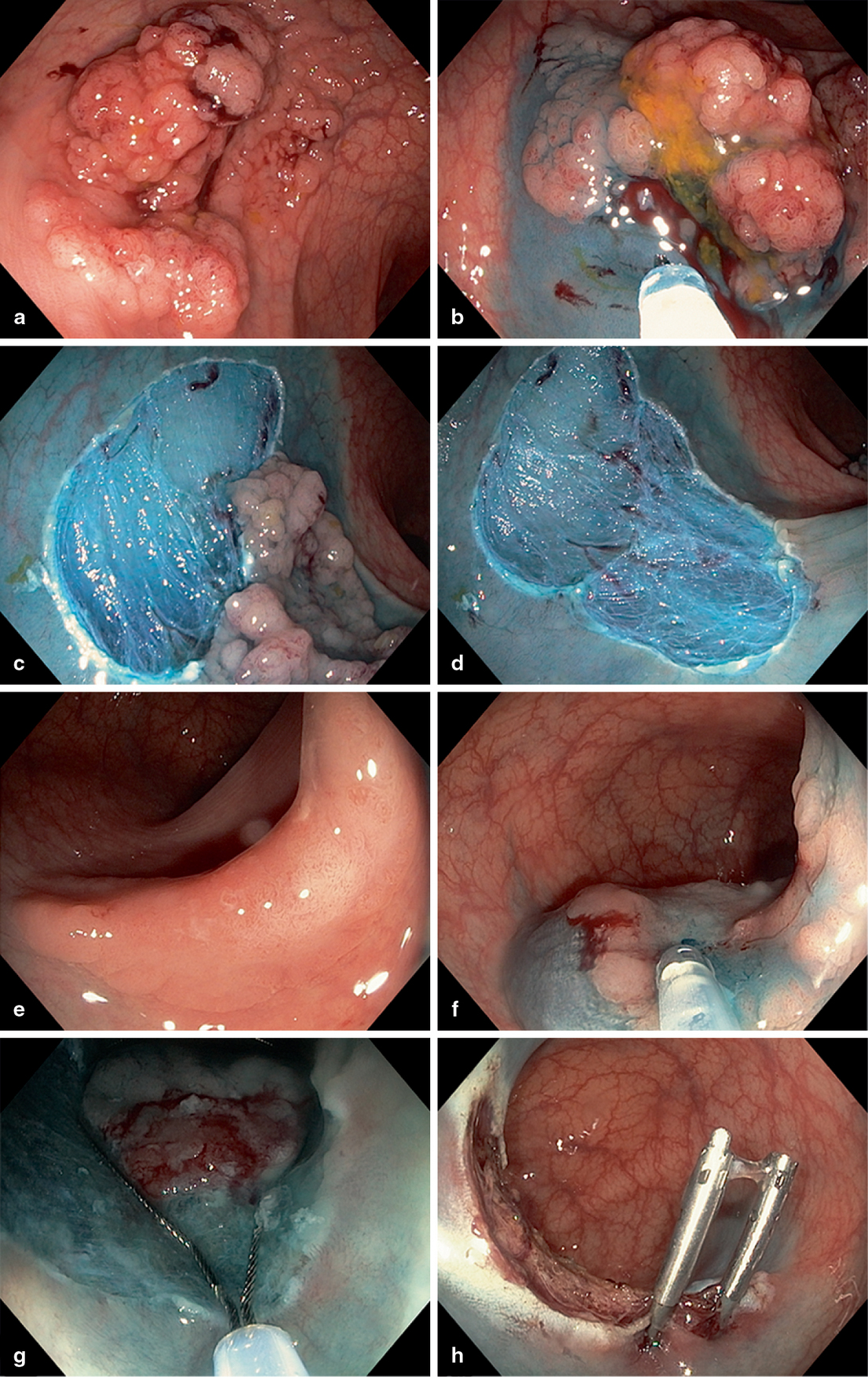

Fig. 5
Analogous signs of submucosal (SM) fibrosis elicited at lesion injection. a Non-lifting with elevation of the surrounding mucosa, the ‘cannyoning’ effect. b The jet sign

Fig. 6
Comparative images of granular and nongranular LST resection. a Forty-five millimetres Granular LST with sessile components ( IIa + Is). b Elevation of the dominant Is component for initial resection. c Resection with margin of normal tissue. d Homogenous blue stained defect. e Thirty millimetres nongranular LST with central depression (IIa + IIc). f Limited elevation of the depressed area which corresponded with adenocarcinoma with superficial submucosal invasion (SMI) on histopathological examination. f Change to small thin-wire snare for resection of poorly lifting component. g Intra-procedural bleeding (IPB) managed with endoscopic clips
Complications
Intra-Procedural Bleeding
Significant IPB occurs in approximately 10–20 % of WF-EMR procedure. It is readily amenable to endoscopic haemostasis. First, the area is irrigated to identify the bleeding point, ideally via a foot pedal operated pump though a dedicated flushing channel. Techniques for haemostasis include: injection of adrenaline, APC, coagulation via snare tip or grasper forceps and endoscopic clipping. The technique of snare tip soft coagulation (STSC) requires 1–2 mm of snare tip to be exposed which is then used to deliver soft coagulation current (80W, effect 4, ERBE VIO) in a targeted fashion to a bleeding site. In a prospective study of 196 patients, a mean lesion size 41.5 mm undergoing WF-EMR, the STSC was effective in 40/44 cases of IPB without complication [82]. For large vessels or pulsatile bleeding, coagulating forceps (utilising the same generator setting, 80W, effect 4) or clips are necessary. These techniques have the disadvantage of additional expense and device exchange time. Clips may also hamper subsequent resection or bury residual adenoma.
Delayed Bleeding
Bleeding is the commonest complication of WF-EMR with the majority occurring within 48 hours of resection. Clinically significant bleeding which is defined as bleeding requiring hospital admission, is seen in 3–7 % of resections in prospective studies [15, 83]. Risk factors for lesions > 20 mm in size include: lesion location in the proximal colon, recent aspirin use and increasing patient age [83, 84]. Most bleeding will settle with conservative management; however, patients with ongoing bleeding or hemodynamic compromise despite resuscitation require intervention. Repeat colonoscopy with endoscopic haemostasis predominately by use of endoscopic clips is usually effective. As the site of bleeding is known and the patient is auto-prepared from the bleeding, additional oral bowel preparation is often not required within this 48 h window. Rarely angiographic embolisation is necessary for cases of failed endoscopic haemostasis or severe bleeding.
Post-Procedural Pain
Mild self-limiting pain is not uncommon after WF EMR and is reduced by use of CO2 insufflation [58]. After WF-EMR for lesions > 30–40 mm, the patients are kept nil by mouth for 2 hours prior to proceduralist review, whilst supine in first-stage recovery. The abdomen is quickly examined and should be soft and non-tender. Patients may then commence clear fluids and be discharged after a further 2 hour period of observation. Persistent pain warrants admission and further evaluation. Computer tomography (CT) is the imaging modality of choice, where perforation is suspected as plain x-ray is relatively insensitive in this setting [85, 86]. Confirmed perforation requires multidiscliplinary management including a colorectal surgical service. Some free air may be seen on CT even after successful endoscopic closure; thus, the management decisions should be driven primarily by the patient’s clinical status.
Perforation and Endoscopic Detection of MP Injury
Although perforation remains a feared complication of EMR, the majority of cases identified at resection may be managed endoscopically [15, 87, 88]. Systematic inspection of the resection defect and the underside of the resected specimen are crucial. Both indigo carmine and methylene blue are avid for the loose connective tissue in the submucosa, thus the typical homogenous blue mat staining of the defect reassures the proceduralist that injury to the MP has not occurred. The MP does not stain and is seen as a white to grey ellipse within the mucosal defect. This may represent a full or partial thickness injury, the latter may present at delayed perforation, if not closed endoscopically. A proportion of non-stained areas will occur due to focally limited dye contact or limited infiltration at SM lift rather than representing muscle injury. TSC allows for a rapid test of staining for interrogation of these areas [72]. The technique involves focused irrigation with dye containing injectate over the unstained area via the injection catheter with the needle retracted. Staining of the submucosa is swift and relatively resistant to water irrigation. Yellow adipose tissue may often be seen in resection defects in the proximal colon and does not represent deep injury.
The target sign is a specific marker for MP resection [87]. It may be seen on the underside of the resection specimen and formed by concentric rings of unstained mucosa and MP (Fig. 7). The mirror target sign may also be appreciated within the colonic resection defect. When identified, it should prompt suctioning of residual faecal fluid and the patient repositioning, so that the defect lies opposite the dependant area to avoid fluid contaminating the defect. Unless there is a clear hole, the residual polyp tissue should be cleared from the adjacent to the defect if feasible, before an attempt at closure as tissue in or under clips may be ‘buried’. Defect closure with endoscopic clips is effective in the majority of cases and can provide closure comparable to surgically placed sutures, as demonstrated in an animal model [89]. Clipping should commence at one end with sequential clips in a ‘zipper’ technique as the placement of a central clip initially can result in unopposed bowed tissue edges (‘dogs ears’). Gentle suction is applied to maximise tissue capture prior to the closure and deployment. Post-procedure these patients are observed and remain nil by mouth for 2 hours before further clinical review by the proceduralist. Patients in whom endoscopic closure is satisfactory and are clinically well without abdominal pain on examination may commence clear liquids orally and after further period of observation may be potentially be discharged home on clear liquid diet the same day.
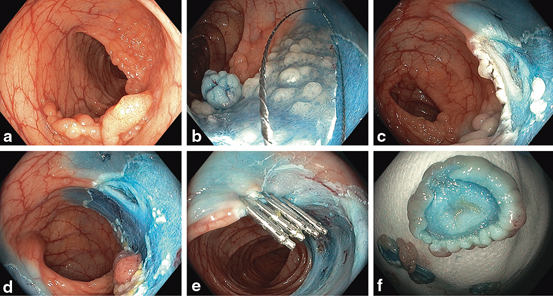

Fig. 7
a Forty-five millimetres sigmoid granular LST (Paris 0-IIa + Is). b Sequential resection. c Mirror target sign indicating MP injury. d Resection of adenomatous tissue adjacent to site of MP injury to avoid buried tissue after closure. e Endoscopic closure with clips and complete resection of the lesion. f Target sign on resection specimen. The patient was admitted overnight for observation and discharged the next day well
Specific Resection Scenarios
Serrated Lesions
The serrated polyp family includes hyperplastic polyps, sessile serrated adenomas/polyps (SSA/Ps) with or without dysplasia and traditional serrated adenomas [19]. Hyperplastic polyps and SSA/Ps without dysplasia are most often flat (0-IIa) by Paris morphology; however, they are not classified by the granular or nongranular surface descriptor. TSAs have the endoscopic appearance and are managed as conventional adenomatous polyps [32]. The majority of large proximal serrated polyps are SSA/Ps [90] which are endoscopically subtle and may has a significant role in the relative failure of colonoscopy to provide similar protection from CRC in the proximal to the distal colon [91–94]. Surveillance guidelines have been updated recognising their significance [95] and their complete endoscopic removal is recommended. Endoscopic predictors of these lesions include adherent mucous or debris [96], pale appearance under NBI [97] and type II-O (open) Kudo-Kimura pit pattern [98]. The low vertical growth and often indistinct margins which make endoscopic detection difficult can lead to challenges during ER . Although the lesion margin may become more obvious after SM injection with dye solution, coagulated margins of normal tissue are difficult to distinguish from the pale tissue of the polyp despite high definition colonoscopes (Fig. 8). A prospective trial of completeness of polyp resection identified SSA/P histology as an independent predictor of residual polyp at polypectomy [99] with residual tissue approaching 50 % for SSAs >10mm in size. Although the incomplete resection rate of SSAs has not been reported upon from a resection series of lesions > 20 mm in size, it is clear that these lesions warrant particular attention when identified. The optimal techniques for complete and safe serrated lesion removal remain unknown and are the subject of ongoing research.
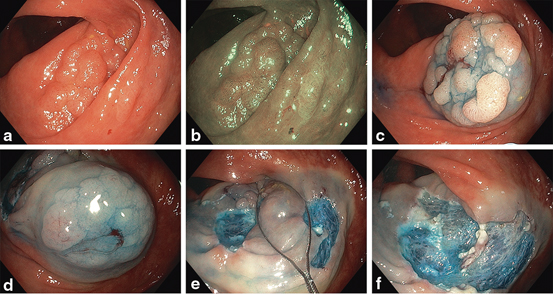

Fig. 8
Thirty millimetres granular LST (Paris 0-IIa) tubular adenoma and adjacent 25 mm (Paris 0-IIa) sessile serrated adenoma in HD WL (a) and NBI (b). Sumucosal injection of the adenomatous (c) and serrated (d) lesions. The SSA margin is clear after injection (e). Resection of rim of intervening normal tissue (f). Resultant wide mucosal defect (g)
Ileocaecal Valve Involvement
The ileocecal valve represents a challenge for complete resection and is a risk factor for recurrence after ER [15]. The margin between villous small intestinal mucosa and adenomatous tissue may be more difficult to appreciate than the junction to colonic mucosa and the smaller ileal lumen may reduce access. Resection may be optimised by use of a clear plastic cap attachment and judicious injection volume along with suitable snare selection.
Periappendiceal
Caecal polyps involving the peri-appendiceal area may be completely resected without perforation providing the appendiceal mucosa can be visualised proximal to the polyp and accessed for snaring after injection. Greater than > 50 % of the appendiceal orifice circumference involvement is a relative contraindication to resection. Prior appendicectomy allows greater confidence during resection in the caecal pole and is a salient history point to note.
Anorectum
Lesions located in the rectum warrant special consideration due to inherent differences in innervation and vascular drainage. For lesions approaching the anal verge, somatic nerve fibres may relay pain post procedure, which can be reduced by addition of long-acting local anaesthesia to the injectate (e.g., 1 % ropivacaine). As the prominent rectal venous network drains directly to the systemic circulation from the distal rectum, prophylactic intravenous broad spectrum antibiotics should be considered to reduce the risk of bacteraemia. The systemic circulatory drainage also dictates that continuous electrocardiograph monitoring be performed when adrenaline and or local anaesthetic is added to the injectate. Access and views may be limited for anorectal lesions and may be improved with a clear cap attachment. Additionally, the confines of the rectum may overcome with use of a gastroscope, with the shorter bending section than a colonoscope resulting in greater manoeuvrability in retroflexion (Fig. 9).
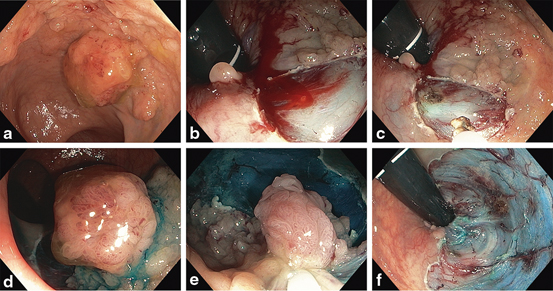





Fig. 9
a Fifty millimetres granular rectal LST with dominant Is nodule (Paris 0-IIa + 0-Is) involving the anorectal margin. b Resection of polyp involving anorectal margin with intra-procedural bleeding (IPB). c Haemostasis after snare tip soft coagulation (STSC). d and e Resection of dominant Is nodule and remaining tissue. f Resultant wide mucosal defect with intact muscularis propria (MP) visible
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








