What the Surgeon Needs to Know: Preoperative Assessment of Patients with Selected Tumors and Organ Transplant Candidates
This section addresses the need to keep the referring physician’s interests and potential future patient management decisions in mind when interpreting an abdominal or pelvic magnetic resonance imaging (MRI) examination. For example, a surgeon referring a patient for MRI evaluation of a tumor already knows that the tumor exists and may already suspect the correct diagnosis. In this setting, the surgeon may primarily be interested in knowing if the patient is a candidate for resection and the obstacles he or she may encounter during surgery. Likewise, providing a report that states essentially “normal liver” for a living-related partial liver donor is not particularly helpful if the vascular and biliary anatomy is not specifically and carefully analyzed.
When confronted with an abdominal or pelvic tumor possibly requiring resection, an attempt should be made to answer the following general questions:
From what organ does the tumor originate? To answer this question, imaging in multiple planes may be necessary, and accounting for all relevant organs is critical. For example, identification of two normal-appearing ovaries dramatically reduces the likelihood that a cystic pelvic mass is of ovarian origin.
Does the tumor extend beyond the serosal layer or capsule of the involved organ? Infiltration or loss of surrounding fat planes or interruption of a normally low signal intensity border suggest extraserosal extension.
Is there evidence of invasion of adjacent organs? An intact fat plane between a tumor and an adjacent organ makes invasion unlikely.
Is there evidence of arterial or venous invasion or encasement? Dedicated vascular sequences may be necessary to answer this question.
Are there enlarged lymph nodes, and, if so, where are they located? Fat-suppressed T2-weighted images are particularly useful for identifying lymph nodes.
Is there evidence of distant metastases? The coronal HASTE breath-hold localizer should be scrutinized for evidence of unsuspected metastases to the spine or lung bases.
Are there any anatomic variants that may present a problem during surgery? Make sure that all relevant vessels or ducts are identified.
To achieve clear surgical margins, how much of the involved organ will need to be resected and how much will remain?
It is unrealistic to expect that a definitive answer can be provided for all of these questions in every case. If any areas of uncertainty exist, the concern can be conveyed to the referring physician with a recommendation for additional imaging (e.g., intraoperative ultrasound or angiography). Surgeons and institutions vary in their criteria for resectability of tumors. Therefore, one should avoid declarations of resectability or unresectability in the dictated report and focus instead on a detailed description of the extent of disease.
Preoperative Assessment of Liver Tumors
Hepatic resection remains the best hope for cure for many patients with malignant and symptomatic benign hepatic tumors. A surgeon referring a patient for evaluation of a liver tumor is interested in the precise location of the tumor, the number and location of additional lesions by segment, the proximity of the tumor to major vascular and biliary structures, the presence of anatomic variants that may complicate
or preclude surgery, and whether sufficient liver mass will remain to sustain life after the tumor is resected.
or preclude surgery, and whether sufficient liver mass will remain to sustain life after the tumor is resected.
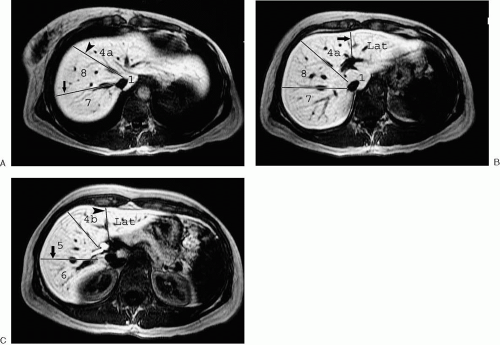
Tumor location is most commonly reported by segment. The traditional segmental anatomy used by radiologists divides the liver into left and right lobes, with each lobe further subdivided into two segments. The plane separating the hepatic lobes is defined by the middle hepatic vein and the gallbladder fossa. The right hepatic lobe is divided into anterior and posterior segments by the right hepatic vein, and the left lobe is divided into medial and lateral segments by the falciform ligament. Even though this approach has the advantage of being relatively simple and reproducible, it does not correspond with the surgeon’s view of hepatic anatomy. Liver surgeons view hepatic anatomy according to segments or subsegments containing specific vascular and biliary branches. This typically involves some variation of the Couinaud system (Fig. 3.198). This is a logical approach, because the surgeon looks for potential planes of resection between the major vascular territories upon which most segmentation systems are based.
Detection of additional lesions is critical in patients being considered for resection of hepatic metastases. Even though gadolinium chelates remain the most commonly used contrast agents for hepatic imaging, there is emerging interest in the use of alternative contrast agents such as ferumoxides for the preoperative detection of hepatic metastases (1,2).
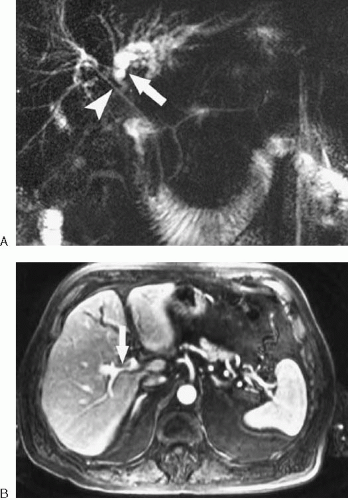
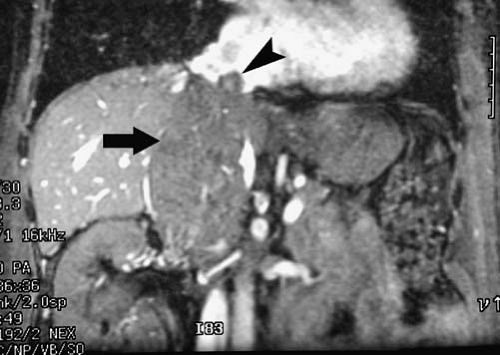
Assessing involvement of biliary and vascular structures is critical to determining resectability of liver tumors. Successful tumor resection is generally not feasible when there is involvement of both right and left hepatic bile ducts, right and left portal veins, right and left hepatic arteries, the main portal vein, the biliary confluence, or the proper hepatic artery (Fig. 3.199). When possible, true vascular invasion or encasement (tumor directly contacts, extends along, narrows,
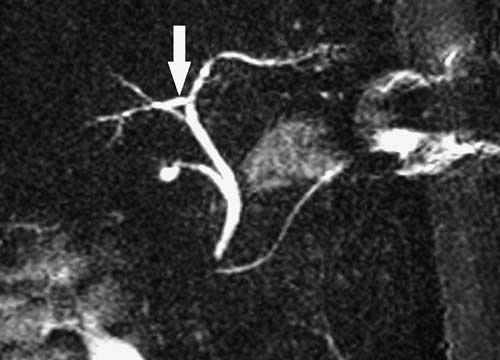
or extends into the vessel) should be distinguished from vessel displacement or compression due to mass effect. Identifying anatomic variants is important to avoid postoperative disruption of the vascular supply or biliary drainage of the remaining liver. For example, if a patient’s right posterior bile duct drains directly into the left hepatic duct, a left hepatectomy could lead to right biliary tract obstruction if the surgeon is unaware of the anatomic variant. (Fig. 3.200). Finally, sufficient liver must remain following tumor resection to sustain life. Therefore, we often provide a volume estimate for the portion of the liver to remain following resection. It may also be useful to provide a percentage estimate of the volume to remain relative to the entire liver. The presence of imaging findings suggesting cirrhosis should be noted, because a greater volume of residual liver may be needed for postoperative survival in this setting. In the future, three-dimensional imaging (3D) techniques will be routinely used to provide quantitative liver resection proposals allowing tumor resection to be based on a preoperative virtual surgery (3).
or extends into the vessel) should be distinguished from vessel displacement or compression due to mass effect. Identifying anatomic variants is important to avoid postoperative disruption of the vascular supply or biliary drainage of the remaining liver. For example, if a patient’s right posterior bile duct drains directly into the left hepatic duct, a left hepatectomy could lead to right biliary tract obstruction if the surgeon is unaware of the anatomic variant. (Fig. 3.200). Finally, sufficient liver must remain following tumor resection to sustain life. Therefore, we often provide a volume estimate for the portion of the liver to remain following resection. It may also be useful to provide a percentage estimate of the volume to remain relative to the entire liver. The presence of imaging findings suggesting cirrhosis should be noted, because a greater volume of residual liver may be needed for postoperative survival in this setting. In the future, three-dimensional imaging (3D) techniques will be routinely used to provide quantitative liver resection proposals allowing tumor resection to be based on a preoperative virtual surgery (3).
Preoperative Assessment of Pancreatic Adenocarcinoma
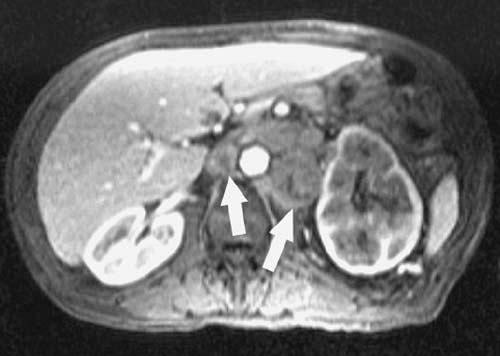
Surgery is the only treatment option with curative potential for patients with pancreatic adenocarcinoma. Therefore, it is important to accurately identify the minority of patients with pancreatic cancer who are candidates for resection. Computed tomography (CT) remains the primary imaging examination for the diagnosis and preoperative evaluation of pancreatic tumors at most centers. However, MRI may be useful in patients for whom contrast-enhanced CT is contraindicated or who require further characterization of an indeterminate CT finding. The accuracy of MRI combined with magnetic resonance cholangiopancreatography (MRCP) and magnetic resonance angiography (MRA) for differentiating benign from malignant pancreatic lesions probably approaches 90%, although the accuracy of MRI for determining tumor nonresectability is somewhat lower (4). Results with MRI for determining resectability are roughly comparable to those reported for CT, although the performance of either modality varies between studies and is likely closely linked to examination quality and interpretive expertise (5,6,7,8).
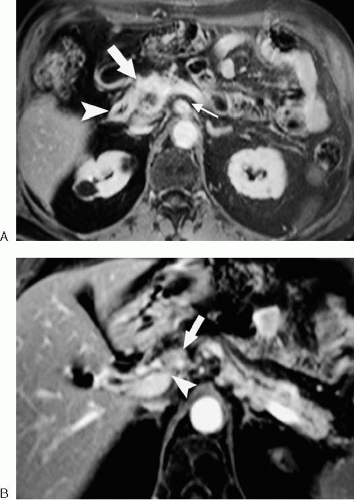
Criteria for resectability of pancreatic adenocarcinoma include absence of tumor invasion or encasement of the celiac axis, superior mesenteric artery, and common hepatic artery (Fig. 201). Tumor encasement of the superior mesenteric
vein (SMV) and main portal vein are relative contraindications to surgical resection. Some surgeons perform a Whipple procedure in the setting of SMV or main portal vein involvement using a vein graft to replace the affected venous segment. Local lymph node involvement is not considered a contraindication to surgery, although it does portend a poor prognosis. Distant tumor spread, such as hepatic or peritoneal metastases, precludes curative surgical resection. (Fig. 3.201).
vein (SMV) and main portal vein are relative contraindications to surgical resection. Some surgeons perform a Whipple procedure in the setting of SMV or main portal vein involvement using a vein graft to replace the affected venous segment. Local lymph node involvement is not considered a contraindication to surgery, although it does portend a poor prognosis. Distant tumor spread, such as hepatic or peritoneal metastases, precludes curative surgical resection. (Fig. 3.201).
One particularly problematic pitfall of pancreatic cross-sectional imaging is the distinction between inflammation and neoplasia. Chronic pancreatitis is notorious for mimicking or obscuring pancreatic tumors, and acute or chronic inflammation may simulate or coexist with adenocarcinoma. For these reasons, radiologists commonly overestimate or underestimate the extent of pancreatic cancer invasion. Acknowledging this potential pitfall to the referring surgeon preoperatively may avoid future persecution at your institutional Tumor Board.
To provide the maximum amount of information to the referring surgeon about the extent of tumor, we recommend that preoperative pancreatic MRI include relatively high-resolution, fat-suppressed, dynamic contrast-enhanced images. Postcontrast images should be obtained at times approximating peak arterial enhancement, peak venous enhancement, and peak pancreatic parenchymal enhancement to maximize conspicuity of the tumor and surrounding vessels. If a thin section, 3D contrast-enhanced acquisition is performed, additional vascular sequences are usually unnecessary, because multiplanar reformations can be constructed to display the vasculature in any plane.
Fat suppression is essential to differentiate normal high signal intensity fat from infiltrating tumor and normal pancreas. A precontrast T1-weighted image without fat suppression may also demonstrate low signal intensity tumor infiltration within the high signal intensity peripancreatic fat. The addition of MRCP sequences is helpful to evaluate the ductal structures. It remains to be seen whether the contrast agent mangafodipir will offer any additional benefit for the diagnosis and staging of pancreatic cancer.
Preoperative Assessment of Presumed Renal Cell Carcinoma
Malignant-appearing renal masses are usually treated surgically. Renal masses may be treated by open or laparoscopic removal or with ablative techniques such as radiofrequency ablation or cryotherapy. Although total nephrectomy is still considered the treatment of choice for most localized renal cell carcinomas, an increasing number of partial nephrectomies and wedge resections are being performed in suitable patients.
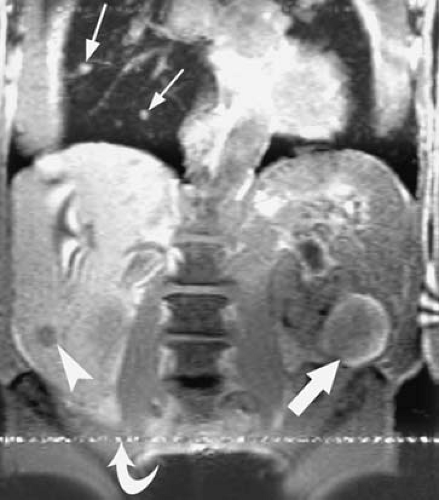
Determining the presence or absence of extracapsular spread (stage 2), renal vein invasion (stage 3a), lymph node involvement (stage 3b), local invasion (stage 4a), and distant metastases (stage 4b) is critical to treatment planning (Figs. 3.202, 3.203). Additionally, tumor size and location, involvement of the renal sinus, vascular anatomy, and status of the contralateral kidney may influence the choice of surgical approaches (e.g., open versus laparoscopic). Extension of intracaval tumor above the confluence of the hepatic veins may necessitate the use of cardiopulmonary bypass, so the presence and extent of intracaval extension must be elucidated (Fig. 3.204).
The evaluation of tumor thrombus in the renal vein and inferior vena cava comprises one of the more common indications for MRI in the preoperative assessment of renal cell carcinoma. For this indication, MRI has been shown to be highly sensitive and specific (9,10). Dark blood, nonenhanced bright blood, and gadolinium-enhanced MRA techniques may all be used to demonstrate the presence and extent
of thrombus. When evaluating the renal vein for tumor, we routinely perform at least two different types of MRA sequences to confirm that the findings are reproducible and not artifactual. Intravenous contrast material is often helpful in distinguishing tumor thrombus from bland thrombus, but the enhancing tumor thrombus may become less conspicuous on venous phase images if the tumor and surrounding blood enhance to a similar degree.
of thrombus. When evaluating the renal vein for tumor, we routinely perform at least two different types of MRA sequences to confirm that the findings are reproducible and not artifactual. Intravenous contrast material is often helpful in distinguishing tumor thrombus from bland thrombus, but the enhancing tumor thrombus may become less conspicuous on venous phase images if the tumor and surrounding blood enhance to a similar degree.
The anatomy of the renal vasculature and collecting systems are well demonstrated with MR techniques and should be assessed in patients with suspected renal cell carcinoma (11,12,13). The sensitivity of contrast-enhanced MRA for the detection of accessory renal arteries exceeds 90%.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree